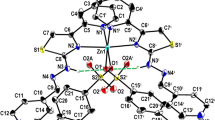
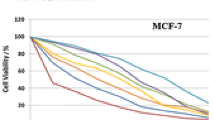
Synthesis of substituted heterocyclic organic ligands and their metal complexes attract more attention in coordination chemistry in recent years owing to its interesting applications in pharmaceutical fields. In this research investigation, we designed a novel thio-substituted pyrazole ligand and synthesized its three novel Co(II), Ni(II), and Zn(II) metal complexes. The ligand, thio-substituted pyrazole is characterized by using single crystal x-ray diffraction data and various physico-chemical measurements such as elemental analysis and UV-Vis, IR, 13C, and 1H NMR, and HRMS spectral techniques. X-ray single crystal measurement of the substituted pyrazole was made on a Brucker AXS Kappa APEX II CCD diffractometer with graphite monochromatic MoK radiation. Metal complexes were characterized by various physico-chemical measurements such as elemental analysis, molar conductance, magnetic susceptibility data, and various spectral data supports. Based on the data obtained from those measurements, an octahedral geometry was assigned to all metal complexes. Furthermore, biological applications of the synthesized complexes were evaluated, mainly their cytotoxic, antitumour, and antimicrobial activities. Zn(II) complex has shown eminent cytotoxic ability, with an IC50 value of 46 μg/mL and its anticancer skill is scrutinized against “Ehrlich’s Ascites Carcinoma” cell line-induced ascites tumour and “Dalton’s Lymphoma Ascites” cell line-induced solid tumour in mice. Additionally, all the metal complexes showed promising antimicrobial skill against selected Gram-positive and Gram-negative bacteria.




Similar content being viewed by others
References
M. Bertuzzi and K. Thøgersen, J. Am. Chem. Soc., 141, 3288 (2019); https: //doi.org/https://doi.org/10.1021/jacs.8b13659
S. Akcha, L. and Hammal, J. Coord. Chem, 11, 6932 (2015); https: //doi.org/https://doi.org/10.1080/00958972.2015.1096932
M. Mohammad, A. R. Gazi, K. Pandav, K, et.al., ACS Omega, 6, 2613 (2021); https: //doi.org/https://doi.org/10.1021/acsomega.0c04775
K. Subin Kumar and K. K. Aravindakshan, Pharm. Chem. J., 55, 1389 (2022); DOI.https://doi.org/10.1007/s11094-022-02585-3
K. Subin Kumar, J. Coord. Chem., 13, 19 (2021); https: //doi.org/https://doi.org/10.1080/00958972.2021.1981885
K. Subin Kumar and K. K. Aravindakshan, Results in Chemistry, 3, 100129 (2021); https: //doi.org/https://doi.org/10.1016/j.rechem.2021.100129
K. Subin Kumar, N. P. Priya and K. K. Aravindakshan, Pharm. Chem. J., 54, 2324 (2021); DOI.https://doi.org/10.1007/s11094-021-02324-0
V. N. Reena, M. Shanasree, K. Subin Kumar, et al., IOP Conf Series: Materials Science and Engineering, 121, 1205 (2022); DOI: https://doi.org/10.1088/1757-899X/1221/1/012051.
K. Subin Kumar and K. K. Aravindakshan, Results in Chemistry, 3, 100129 (2021); https: //doi.org/https://doi.org/10.1016/j.rechem.2021.100129
P. P. Soufeena, K. Subin Kumar and K. K. Aravindakshan, Materials Today Proceed., 5, 16790 (2018); https: //doi.org/https://doi.org/10.1016/j.matpr.2018.06.017
T. I. Chaban and Y. E. Matiichuk, Acta Chimica Slovenica, 67, 1035 (2020); http: //dx.doi.org/https://doi.org/10.17344/acsi.2019.5439.
N. T. Pokhodylo, V. S. Matiychuk and N. D. Obushak, Chem. Heterocycl. Comp., 45, 483 (2009); https: //doi.org/https://doi.org/10.1007/s10593-009-0287-6.
T. Chaban, V. Matiychuk, A. Mahlovanyy, et al., Biointerface Res. Appl. Chem., 10, 5944 (2020); https: //doi.org/https://doi.org/10.33263/BRIAC104.944950.
O. Tymoshuk, L. Oleksiv, L. Khvalbota, et al., Acta Chimica Slovenica, 66, 62 (2019); https: //doi.org/https://doi.org/10.17344/acsi.2018.4448.
Y. Matiichuk, Y. Ostapiuk and T. Chaban, Biopolymers & Cell, 36, 74 (2020); http: //dx.doi.org/https://doi.org/10.7124/bc.000A22.
T. I. Chaban and Y. E. Matiichuk, Russ. J. Org. Chem., 56, 813 (2020); https: //doi.org/https://doi.org/10.1134/S1070428020050139.
K. Subin Kumar and V. N. Reena, Materials Today Proceed., 18, 2987 (2022); https: //doi.org/https://doi.org/10.1016/j.matpr.2022.03.704
T. Chaban and Y. Matiichuk, Biointerface Res. Appl. Chem., 11, 10955 (2021); https: //doi.org/https://doi.org/10.33263/BRIAC113.1095510967.
T. I. Chaban and V. S. Matiychuk, Russ. J. Gen. Chem., 90, 1578 (2020); https: //doi.org/https://doi.org/10.1134/S1070363220080290.
Z. Chulovska and I. Drapak, et al., Eur. Chem. Bull., 10, 147 (2021); https: //doi.org/https://doi.org/10.17628/ecb.2021.10.147-154.
D. Dou, G. He, S. R. Mandadapu, et al., Bioorg. & Med. Chem. Lett., 22, 377 (2012); http: //dx.doi.org/https://doi.org/10.1016/j.bmcl.2011.10.122.
N. Agrawal, Curr. Chem. Lett, 10, 119 (2021); https: //doi.org/https://doi.org/10.5267/j.ccl.2020.11.002.
R. P. Singh, M. N. Aziz, D. Gout, et al., Bioorg. Med. Chem., 27, 5047 (2019); https: //doi.org/https://doi.org/10.1016/j.bmc.2019.115047.
A. Türe, M. Ergül, A. Altun, and I. Küçükgüzel, Mol. Divers, 1, 0087 (2020); https: //doi.org/https://doi.org/10.1007/s11030-020-10087-1.
M. Lelyukh, I. Demchuk, S. Harkov, et al., Biointerface Res. Appl. Chem. 10, 5960 (2020); https: //doi.org/https://doi.org/10.33263/BRIAC104.960971.
M. Lelyukh, M. Martynets and M. J. Kalytovska, Appl. Pharm. Sci., 10, 151 (2020); https: //doi.org/https://doi.org/10.7324/JAPS.2020.1010016.
K. Subin Kumar and V. N. Reena, Materials Today Proceed., 62, 8 (2022); https: //doi.org/https://doi.org/10.1016/j.matpr.2022.03.704
Acknowledgements
Authors expresses their gratitude to Amala Cancer Research Centre and STIC Cusat, Cochin for conducting elemental analysis and spectral analysis.
Funding agency
No funding agency available.
Conflict of interest Statement
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, K.S., Reena, V.N. Crystal Structure, Anticancer, and Antimicrobial Evaluation of a Novel Substituted Pyrazole Ligand and Its Co(II), Ni(II), and Zn(II) Complexes. Pharm Chem J 57, 1251–1259 (2023). https://doi.org/10.1007/s11094-024-03031-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-024-03031-2