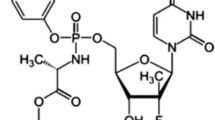
Favipiravir (FAV) is an antiviral drug in tablets, which has been originally intended for the treatment of influenza and determined by methods based mostly on liquid chromatography. Recently, FAV has been also tried for the treatment of COVID-19 in many countries, Here we present for the first time in the literature a facile and sensitive stability-indicating UV-spectrophotometric method of determining FAV that is effective on a micromolar scale. This green method was developed in aqueous drug solutions. Acidic, basic, oxidative degradation at room temperature as well as thermal degradation studies at 60°C were performed and fully validated in accordance with ICH guidelines, with 230 and 323 nm detection wavelengths used in linearity studies. The developed method was linear at concentrations between 5.0 – 30.0 μg/mL with correlation coefficient > 0.99. FAV concentrations of 1.20 and 3.70 μg/mL were calculated as LOD and LOQ levels, respectively. Recovery results were found within satisfactory precision values of 99.6 – 100.88%. The developed method gives results statistically comparable with those obtained by the HPLC technique. Due to the elimination of FAV mainly in its intact form when applied at clinical doses, this method can easily be applied for clinical and routine analyses as well as in quality control laboratories.


Similar content being viewed by others
References
D. H. Goldhill, A. J. W. Te Velthuis, R. A. Fletcher, et al., Proc. Natl. Acad. Sci. U. S. A., 115, 11613 – 11618 (2018).
WHO Covid-19 (2020); https://www.who.int/emergencies/diseases/novel-coronavirus-20193.
U. Agrawal, R. Raju and Z. F. Udwadia, Med. J. Armed. Forces. India, 76, 370 – 376 (2020).
L. Naesens, L. W. Guddat, D. T. Keough, et al., Mol. Pharmacol., 84, 615 – 629 (2013).
V. Madelain, T. H. Nguyen, A. Olivo, et al., Clin. Pharmacokinet., 55, 907 – 923 (2016).
Y. Furuta, B. B. Gowen, K. Takahashi, et al., Antivir. Res., 100, 446 – 454 (2013).
O. Ayten, C. Ozdemir, U. A. Akturk and N. Sen, Eurasian J. Pulmonol., 22, 35 – 44 (2020).
Ý. Bulduk, Acta Chromatogr., 33, 209 – 215 (2021).
T. H. Nguyen, J. Guedj, X. Anglaret, et al., PLoS Negl. Trop. Dis., 11, e0005389 (2017).
V. Madelain, J. Guedj, T. H. T. Nguyen, et al., Antimicrob. Agents Chemother., 61, e01305 – 16 (2017).
M. I. Morsy, E. G. Nouman, Y. M. Abdallah, et al., J. Pharm. Biomed. Anal., 199, 114057 (2021).
R. R. Mamdouh, K. A. Badr, N. S. Abdel-Naby, and M. M. Ayyad, Biomed. Chromatog., 35, e5098 (2021).
I. E. Mikhail, H. Elmansi, F. Belal, and A. E. Ibrahim, Microchem. J., 165, 106189 (2021).
S. M. Megahed, A. A. Habib, S. F. Hammad, and A. H. Kamal, Spectrochim. Acta A Mol. Biomol. Spectrosc., 249, 119241 (2021).
S. Allahverdiyeva, O. Yunusoğlu, Y. Yardım, and Z. Şentürk, Anal. Chim. Acta, 1159, 338418 (2021).
M. A. Mohamed, G. M. G. Eldin, S. M. Ismail, et al., J. Electroanal. Chem., 895, 115422 (2021).
M. Bakshi and S. Singh, J. Pharm. Biomed. Anal., 28, 1011 – 1040 (2002).
I. C. H. S. Committee, ICH Q2B validation of analytical procedures: methodology, Eur. Agency Eval. Med. Prod. Int. Comm. Harmon., London (1996).
N. Tkachenko, Optical Spectroscopy, Methods and Instrumentations, Elsevier, Amsterdam (2016).
Handbook of Pharmaceutical Excipients, Eighth Edition, ed. by Paul J. Sheskey, Walter G. Cook and Colin G. Cable, Pharmaceutical Press and American Pharmacists Association (2017).
Acknowledgements
The authors would like to thank Biophore India Pharmaceuticals for allowing them to use the HPLC method developed in this work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evcil, I., Pehlivanoglu, H., Boğa, M. et al. A Green, Validated and Stability Indicating UV Spectrophotometric Determination And HPLC Comparison of Covid-19 Drug Favipiravir in Tablets. Pharm Chem J 57, 584–589 (2023). https://doi.org/10.1007/s11094-023-02927-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02927-9