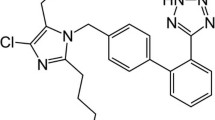
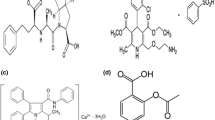
Cardiovascular drugs are the main components of many formulations in marketplace. Herein, a fast high-performance liquid chromatographic assay was developed for the determination of pharmaceutical preparations containing antihypertensive agents including Atenolol (ATE), Hydrochlorothiazide (HCT), Ramipril (RAM), Amlodipine (AML), Losartan (LOS) and Telmisartan (TEL). Optimum separation was achieved using RP-C8 Waters Symmetry column (3.5 μm, 4.6 mm × 150 mm) and a mobile phase composed of 25 mM potassium phosphate buffer (pH 4.5 ± 0.1) and acetonitrile in gradient elution mode at 1.0 mL/min flow rate and 230 nm detection wavelength. Analysis was achieved in less than 9 min. The method was fully validated according to ICH guidelines. Linearity was achieved over the range of 10.0 – 60.0 μg/mL for ATE, LOS, Tel, 5.0 – 50.0 μg/mL for HCT, AML, and 1.0 – 11.0 μg/mL for RAM with acceptable accuracy, precision and limits of detection (< 2.0 μg/mL). Method robustness was assessed using small changes in buffer pH, acetonitrile ratio of the mobile phase, flow rate and detection wavelength. The proposed method was successfully applied to the analysis of their dosage forms combinations. Statistical comparison between the proposed and official methods was performed. The optimized method offers an economic chromatographic tool for the routine analysis of cardiovascular drugs in pharmaceutical quality control laboratories.



Similar content being viewed by others
References
R. D. Howland, M. J. Mycek, R. A. Harvey, and P. C. Champe, Lippincott’s Illustrated Reviews: Pharmacology, Lippincott Williams & Wilkins, Philadelphia (2006).
S. C. Sweetman, Martindale : The Complete Drug Reference, Pharmaceutical Press, London (2009).
A. C. Moffat, M. D. Osselton, B.Widdop, and J.Watts, Clarke’s analysis of drugs and poisons, Pharmaceutical Press, London (2011), Vol. 3.
K. K. Chaitanya, D. G. Sankar, and D. S. Israel, Global Treat. Pharm. Sci., 4, 1144 – 1152 (2013).
L. Li, C. Lai, X. Xuan, et al., J. Chromatogr. Sci., 54(8), 1415 – 1420 (2016).
P. S. Tungenwar, S. Ahmad, V. M. Shastry, and T. Mujawar, J. Pharm. BioSci., 5(2), 14 – 19 (2017).
A. R. Tengli, B. Gurupadayya, and N. Soni, Int. J. Chem. Anal. Sci., 4(1), 33 – 38 (2013).
M. Baing, V. Vaidya, R. Sane, et al., Chromatographia, 64(5), 293 – 296 (2006).
D. D. Rao, N. V. Satyanarayana, S. S. Sait, et al., Chromatographia, 70(3 – 4), 647 – 651 (2009).
M. K. Shabana, D. Chapala, and P. S. Babu, Int. Res. J. Pharm., 4(8), 177 – 183 (2013).
I. Salama, Bull. Fac. Pharm. Cairo Univ., 49(1), 19 – 24 (2011).
V. P. Kurade, M. G. Pai, and R. Gude, Indian J. Pharm. Sci., 71(2), 148 – 151 (2009).
P. Wal, R. Tiwari, A. Wal, and G. Tiwari, Int. J. Appl. Pharm., 10(4), 51 (2018).
R. Dubey and M. Ghosh, Sci. Pharm., 83(1), 107 – 124 (2015).
V. Gupta, R. Jain, O. Lukram, et al., Talanta, 83(3), 709 – 716 (2011).
S. Magiera, J. Chromatogr. B, 938, 86 – 95 (2013).
A. K. Palakurthi, T. Dongala, and S. R. Yadlapalli, Sep. Sci. Plus, 3(6), 191 – 199 (2020).
K. S. Lakshmi and S. Lakshmi, J. Anal. Methods Chem., 2012, 108281 (2012).
R. A. Shaalan, T. S. Belal, F. A. El Yazbi, and S. M. Elonsy, Bull. Fac. Pharm. Cairo Univ., 52(2), 225 – 237 (2014).
H. M. Ahmed, T. S. Belal, R. A. Shaalan, et al., Acta Chromatogr., 32(4), 219 – 227 (2020).
A. Modroiu, G. Hancu, R. A. Vlad, et al., Curr. Issues Pharm. Med. Sci., 29, 42 – 46 (2016).
British Pharmacopoeia, British Pharmacopoeial Commission, The Stationery Office, London (2016).
ICH Harmonized Tripartite Guidelines, Validation of Analytical Procedures: Text and Methodology Q2(R1), International Conference on Harmonization of Technical Requirments for Registeration of Pharmaceuticals for Human Use, Geneva, (2005).
S. Moldoveanu and V. David, Selection of HPLC Method in Chemical Analysis, Elsevier (2016).
Validation of Chromatographic Methods, Center for Drug Evaluation and Research (CDER), Washington (1994).
J. Nikelly, HPLC and CE: Principles and Practice, Academic Press, San Diego (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saeed, S., Nadim, A.H., Yehia, A.M. et al. A Generic High-Performance Liquid Chromatographic Method for Simultaneous Determination of Six Cardiovascular Drugs: Method Optimization and Application to Various Pharmaceutical Formulations. Pharm Chem J 57, 138–145 (2023). https://doi.org/10.1007/s11094-023-02861-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02861-w