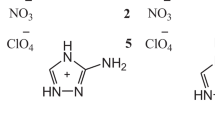
Effective drugs with antiaggregant and anticoagulant activity are known to possess side effects. It was hypothesized that compounds synthesized from natural amino acids would possess these pharmacological activities without many of the side effects. In this regard, a number of amino-acid salts of alkali and alkaline-earth metals were synthesized and studied. The newly synthesized compounds were identified using PMR spectra, IR spectroscopy, and elemental analysis. Laboratory studies carried out with isolated blood of healthy volunteers identified seven compounds exhibiting antiaggregant properties. The antiaggregant effects of these compounds on the maximum amplitude of aggregation was comparable to that of acetylsalicylic acid and exceeded it in terms of inhibition of the platelet release reaction. Computer simulation of the interaction of the most active amino-acid salts with the surface of cyclooxygenase confirmed their possible participation in the inhibition of this enzyme.



Similar content being viewed by others
References
A. C. Vlot, D. A. Dempsey, and D. F. Klessig, Annu. Rev. Phytopathol., 47, 177 – 206 (2009); https://doi.org/10.1146/annurev.phyto.050908.135202.
J. Vane, Nature (London), New Biol., 231, 232 – 235 (1971); https://doi.org/10.1038/newbio231232a0.
Y. H. Mekaj, F. T. Daci, and A. Y. Mekaj, Ther. Clin. Risk Manage., 11, 1449 – 1456 (2015); https://doi.org/10.2147/TCRM. S92222.
J. P. Collet, H. Thiele, E. Barbato, et al., Eur. Heart J., 42(14), 1289 – 1367 (2021); https://doi.org/10.1093/eurheartj/ehaa575.
E. H. Awtry and J. Loscalzo, Circulation, 101, 1206 – 1218 (2000); https://doi.org/10.1161/01.CIR.101.10.1206.
D. Ekinci, M. Senturk, and O. I. Kufrevioglu, Expert Opin. Ther. Pat., 21, 1831 – 1841 (2011); https://doi.org/10.1517/13543776.2011.636354.
O. I. Kulikova, S. L. Stvolinsky, V. A. Migulin, et al., Daru, J. Fac. Pharm., Tehran Univ. Med. Sci., 28(1), 119 – 130 (2020); https://doi.org/10.1007/s40199-019-00323-x.
Z. Yin, W. Hu, W. Zhang, et al., Amino Acids, 52(9), 1227 – 1261 (2020); https://doi.org/10.1007/s00726-020-02887-4.
S. Broer and A. Broer, Biochem. J., 474, No. 12, 1935 – 1963 (2017); https://doi.org/10.1042/BCJ20160822.
R. G. Kadyrova, G. F. Kabirov, and R. R. Mullakhmetov, Synthesis and Properties of Complex Salts of Biogenic Acids of Macro- and Microelements [in Russian], Kazan Gos. Energ. Univ., Kazan’ (2016).
V. A. Klimova, Basic Microanalytical Methods of Organic Compounds [in Russian], Khimiya, Moscow (1975), pp. 51 – 56.
G. V. R. Born, J. Physiol., 162, 67 – 68 (1962).
D. A. Abaimov, L. R. Spavronskaya, A. A. Shabalina, et al., Khim.-farm. Zh., 53(1), 52 – 57 (2019); Pharm. Chem. J., 53(1), 65 – 70 (2019); https://doi.org/10.1007/s11094-019-01957-6.
A. N. Mironov (ed.), Handbook for Preclinical Drug Studies [in Russian], Vol. 1, Grif i K, Moscow (2013).
D. Schneidman-Duhovny, Y. Inbar, R. Nussinov, and H. J. Wolfson, Nucleic Acids Res., 33, 363 – 367 (2005).
D. Picot, P. J. Loll, and R. M. Garavito, Nature, 67, 43 – 49 (1994).
G. J. Roth, N. Stanford, and P. W. Majerus, Proc. Natl. Acad. Sci. USA, 72, 3073 – 3076 (1975).
M. Miciaccia, B. D. Belviso, M. Iaselli, et al., Sci. Rep., 11(1), Art. No. 4312 (2021); https://doi.org/10.1038/s41598-021-83438-z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 11, pp. 21 – 27, November, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gurevich, K.G., Urakov, A.L., Purygin, P.P. et al. Synthesis and Antiaggregant and Anticoagulant Activity of Amino-Acid Salts and Computer Simulation of the Interaction of Their Structures with the Surface of Cyclooxygenase. Pharm Chem J 56, 1451–1456 (2023). https://doi.org/10.1007/s11094-023-02812-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02812-5