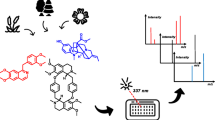
Tandem mass spectrometry (HPLC-MS/MS) was used to analyze biologically active compounds in extracts of the tuber and roots of Rhodiola rosea (family Crassulaceae) and in the pharmaceutical formulation Multiphytoadaptogen (MPA). Chromatography was run on Acquity UPLC BEH C18 columns with gradient elution. A TSQ Vantage ternary quadrupole mass spectrometer with electrospray ionization was used. MPA, like the Rhodiola rosea tuber/root extract, was found to contain salidroside, rosavin, rosarin, rosiridin, and rhodionin. These results can be used for standardization, identification, and quality control of phytocomplexes containing these Rhodiola rosea compounds to identify grounds for the mechanisms of the biological actions of MPA and studying its novel properties, taking account of the components identified. The PASS computer program was used for in silico evaluation of the spectra of the antitumor activities of these compounds. Antitumor effects were predicted with high probability for all five phytocomponents; the specific characteristic of the actions of individual compounds suggested that combinations might be used for treating a wide spectrum of malignant neoplasms.



Similar content being viewed by others

References
O. A. Bocharova, R. V. Karpova, E. V. Bocharov, et al., Ros. Bioterapevt. Zh., 19(4), 22 – 31 (2020).
O. N. Kurennaya, R. V. Karpova, O. A. Bocharova, et al., Genetika, 49(12), 1364 (2013); Russ J. Genetics, 49(12), 1190 (2013).
O. A. Bocharova, V. B. Matveev, R. V. Karpova, et al., Bull. Exp. Biol. Med., 141(5), 616 – 619 (2006).
O. A. Bocharova, M. M. Pozharitskaya, T. L. Chekalina, et al., Bull. Exp. Biol. Med., 138(6), 578 – 583 (2004).
E. V. Bocharov, V. G. Kucheryanu, G. N. Kryzhanovsky, et al., Bull. Exp. Biol. Med., 141(5), 560 (2006).
O. A. Bocharova, M. I. Davydov, A. A. Klimenkov, et al., Bull. Exp. Biol. Med., 148(1), 82 (2009).
E. V. Bocharov, O. A. Bocharova, R. V. Karpova, et al., Ros. Bioterapevt. Zh., 17(2), 69 (2018).
O. P. Sheichenko, O. A. Bocharova, B. A. Krapivkin, et al., Vopr. Biol. Med. Farm. Khim., 10(52) (2012).
O. A. Bocharova, I. V. Kazeev, V. E. Shevchenko, et al., Teor. Osnov. Khim. Tekhnol., 55(6), 780 – 792 (2021).
I. V. Kazeev, O. A. Bocharova, V. E. Shevchenko, et al., Teor. Osnov. Khim. Tekhnol., 54(6), 733 – 737 (2020).
A. S. Saratikov and E. A. Krasnov, Rhodiola Rosea – a Valuable Medicinal Herb: (Golden Root), TOnsk University Press, Tomsk (1987).
G. G. Zapesochnaya and V. A. Kurkin, Khim. Prirodn. Soedin., 6, 723 – 727 (1982).
G. G. Zapesochnaya and V. A. Kurkin, Khim. Prirodn. Soedin., 1, 23 – 32 (1983).
V. A. Kurkin, G. G. Zapesochnaya, and A. N. Shchavlinskii, Khim. Prirodn. Soedin., 5, 632 – 636 (1985).
Y. Li, V. Pham, M. Bui, and L. Song, Curr. Pharmacol. Rep., 3(6), 384 – 395 (2017); doi: 101007 / s40495-017-0106-1.
A. Panossian, Ann. NY Acad. Sci., 1401, No. 1, 49 – 64 (2017).
Z. L. Liang, X. Y. Zhang, F. Wang, et al., Medicine (Baltimore), 97(39), e11886 (2018); doi: https://doi.org/10.1097/MD0000000000011886.
A. Booker, B. Jalil, D. Frommenwiler, et al., Phytomedicine, 23(7), 754–762 (2016); doi: https://doi.org/10.1016/jphymed201510006.
Y. C. Ma, X. Q. Wang, F. Hou, et al., J. Pharm. Biomed. Anal., 55(5), 908 – 915 (2011); doi: https://doi.org/10.1016/jjpba201103013.
V. A. Kurkin and T. K. Ryazanova, Khim.-Farm. Zh., 55(8), 40 – 44 (2021); Pharm Chem J, 55(8), 793 – 797 (2021).
A. Q. Sun, X. L. Ju, J. Chin, Integr. Med, 27(2), 153 – 160 (2021); doi: https://doi.org/10.1007/s11655-020-3190-8.
L. Yang, Y. Yu, Q. Zhang, et al., Artif. Cells Nanomed. Biotechnol., 47(1), 3500 – 3510 (2019); doi: https://doi.org/10.1080/2169140120191652626.
S. Y. Ding, M. T. Wang, D. F. Dai, et al., Biochem. Biophys. Res. Commun., 527(4), 1057 – 1063 (2020); doi: https://doi.org/10.1016/jbbrc202005066.
M. Ren,W. Xu, T. Xu, Artif. Cells Nanomed. Biotechnol., 47(1), 1014 – 1021 (2019); doi: https://doi.org/10.1080/2169140120191584566.
A. S. Marchev, P. Dimitrova, and I. K. Koycheva, Food Chem. Toxicol., 108 (Part B), 419 – 428 (2017); doi: https://doi.org/10.1016/jfct201702009.
S. K. J. Magani, S. D. Mupparthi, B. P. Gollapalli, et al., Curr. Drug Metab., 21(7), 512 – 524 (2020); doi: https://doi.org/10.2174/1389200221666200610172105.
S. Sun, Q. Tuo, D. Li, et al., Evid. Based Complement Alternat. Med., 2020, 9568647 (2020); doi: https://doi.org/10.1155/2020/9568647.
K. Khanna, K. P. Mishra, L. Ganju, et al., Biomed. Pharmacother., 87, 496 – 502 (2017); doi: https://doi.org/10.1016/jbiopha201612132.
R. Li and J. Chen, Oxid. Med. Cell. Longev., 2019, 9341018 (2019); doi: https://doi.org/10.1155/2019/9341018.
W. Zhou, K. Chen, Q. Lu, et al., Chem. Biodivers., 17(12), e2000652 (2020); doi: https://doi.org/10.1002/cbdv202000652.
L. Mácsai, Z. L. Datki, D. Csupor, et al., Evid. Based Complement Alternat. Med., 2018, 3690683 (2018); doi: https://doi.org/10.1155/2018/3690683.
E. V. Bocharov, O. A. Bocharova, R. V. Karpova, et al., Ros. Bioterapevt. Zh., 17(2), 69 – 78 (2018).
O. A. Bocharova, R. V. Karpova, and E. V. Bocharov, Bull. Exp. Biol. Med., 159(5), 655 – 657 (2015).
E. V. Bocharov, V. G. Kucheryanu, and G. N. Kryzhanovskii, Bull. Exp. Biol. Med., 141(5), 560 – 563 (2006); doi: https://doi.org/10.1007/s10517-006-0220-2.
E. V. Bocharov, V. G. Kucheryanu and I. A. Ivanova-Smolenskaya, Bull. Exp. Biol. Med., 149(6), 682 – 684 (2010).
E. V. Bocharov, R. V. Karpova, I. V. Kazeev, et al., Pat. Fiziol. Éksperim. Ter., 57(3), 55 – 58 (2013).
R. V. Karpova, E. V. Bocharov, I. V. Kazeev, et al., Pat. Fiziol. Éksperim. Ter., 57(4), 51 – 54 (2013).
O. A. Bocharova, M. I. Davydov, A. A. Klimenkov, et al., Bull. Exp. Biol. Med., 148(1), 82 – 85 (2009).
O. A. Bocharova, R. V. Karpova, V. A. Golubeva, et al., Bull. Exp. Biol. Med., 128(4), 1005 – 1008 (1999).
O. A. Bocharova, R. V. Karpova, E. V. Bocharov, et al., Ros. Bioterapevt. Zh., 19(4), 35 – 44 (2020).
A. A. Lagunin, R. K. Goel, D. Y. Gawande, et al., Nat. Prod. Rep., 31(11), 1585 – 1611 (2014).
D. Y. Gawande, D. Druzhilovsky, R. C. Gupta, et al., J. Ethnopharmacol., 202(18), 97 – 102 (2017).
A. K. Uéili, A. O. Ponkratova, A. A. Orlova, et al., Khim.- Farm. Zh., 55(3), 22 – 27 (2021); Pharm Chem J., 55(3), 253 – 258 (2021).
Acknowledgements
This study was partially funded by grants from the Commission for Biomedical Innovations and Technologies, Russian Federation Ministry of Science, and the Basic Long-Term Scientific Research Program of the Russian Federation (2021 – 2030).
Conflict of interests. The authors declare that they have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 56, No. 1, pp. 32 – 38, January, 2022.
Rights and permissions
About this article
Cite this article
Bocharova, O.A., Kazeev, I.V., Shevchenko, V.E. et al. A Potential Method for Standardization of Multiphytoadaptogen: Tandem Mass Spectrometry for Analysis of Biologically Active Substances from Rhodiola rosea. Pharm Chem J 56, 78–84 (2022). https://doi.org/10.1007/s11094-022-02607-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02607-0