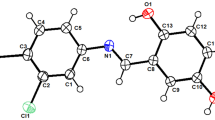
Benzylsulfamic acid has been prepared and introduced as a new heterogeneous acid catalyst. This reagent was used for the synthesis of new bis(indolyl)methanes 3a – 3f via reaction of bis-aldehydes 1 with indoles 2 at 100°C. All reactions were performed under solvent-free conditions with high to excellent yields. The synthesized bis(indolyl)methanes were evaluated for their antibacterial and antifungal activities.



Similar content being viewed by others
References
M. C. Bell, P. Crowley-Nowick, H. L. Bradlow, et al., Gynecol. Oncol., 78, 123 – 129 (2000).
J. J. Michnovicz and H. L. Bradlow, Nutr. Cancer,16, 59 – 66 (1991).
G. R. Humphrey and J. T. Kuethe, Chem. Rev.,106, 2875 – 2911 (2006).
K. Sujatha, P. T. Perumal, D. Muralidharan and M. Rajendran, Indian J. Chem.,48, 267 – 269 (2009).
S. H. Benabadji, R. Wen, J. Zheng, et al., Acta. Pharmacol. Sinica.,25, 666 – 671 (2004).
G. Sivaprasad, P. T. Perumal, V. R. Prabavathy and N. Mathivanan, Bioorg. Med. Chem. Lett.,16, 6302 – 6305 (2006).
A. Kamal, M. N. A. Khan, K. S. Reddy, et al., J. Enzyme. Inhib. Med. Chem.,24, 559 – 565 (2009).
K. R. M. Naidu, S. I. Khalivulla, S. Rasheed, et al., Int. J. Mol. Sci.,14, 1843 – 1853 (2013).
C. Hong, G. L. Firestone and L. F. Bjelanes, Biochem, Pharmacol., 63, 1085 – 1097 (2002).
T. Osawa and M. Namiki, Tetrahedron Lett.,24, 4719 – 4722 (1983).
W. Kassouf, S. Chintharlapalli, M. Abdelrahim, et al, Cancer Res.,66, 412 – 418 (2006).
J. S. Glasby, Encyclopedia of Aalkaloids, New York: Plenum Press (1975).
M. Kirkus, M. H. Tsai, J. V. Grazulevicius, et al., Synth. Met.,159, 729 – 734 (2009).
X. He, S. Hu, K. Liu, et al., Org. Lett.,8, 333 – 336 (2006).
R. Martinez, A. Espinosa, A. Tarraga and P. Molina, Tetrahedron,64, 2184 – 21891 (2008).
B. S. Liao, J. T. Chen and S. T. Liu, Synthesis,20, 3125 – 3128 (2007).
X. F. Zeng, S. J. Ji and S. Y. Wang, Tetrahedron,61, 10235–10241 (2005).
J. S. Yadav, B. V. S. Reddy, V. Sunitha and K. S. Reddy, Adv. Synth. Catal.,345, 1203 – 1206 (2003).
A. K. Chakraborti, S. R. Roy, D. Kumar and P. Chopra, Green Chem.,10, 1111 – 1118 (2008).
K. Rad-Moghadam and M. Sharifi-Kiasaraie, Tetrahedron,69, 8816 – 8820 (2009).
P. J. Das and J. Das, Tetrahedron Lett.,53, 4718 – 4720 (2012).
H. Firouzabadi, N. Iranpoor and A. A. Jafari, J. Mol. Catal.,244, 168–172 (2006).
V. Jhansi Rani, K. Veena Vani and C. Venkata Rao, Synth. Commun.,42, 2048–2057 (2012).
A. Karam, J. C. Alonso, T. I. Gerganova, et al., Chem. Commun., 45, 7000 – 7002 (2009).
M. M. Heravi, K. Bakhtiari, A. Fatehi and F. F. Bamoharram, Catal. Commun., 9, 289 – 292 (2008).
H. Veisi, B. Maleki, F. H. Eshbala, et al., RSC Adv.,4, 30683 – 30688 (2014).
H. Firouzabadi, N. Iranpoor, M. Jafarpour and A. Ghaderi, J. Mol. Catal. A: Chem.,253, 249 – 251 (2006).
G. V. M. Sharma, J. J. Reddy, P. S. Lakshim and P. R. Krishna, Tetrahedron Lett.,45, 7729 – 7732 (2004).
M. A. Zolfigol, P. Salehi, M. Shiri, et al., Mol. Divers., 2, 203 – 207 (2008).
B. M. Reddy, P. M. Sreekanth and P. Lakshmanan, J. Mol. Catal. A. Chem.,237, 93 – 100 (2005).
K. Niknam, M. A. Zolfigol, T. Sadabadi and A. Nejati, J. Iran. Chem. Soc.,3, 318–322 (2006).
M. Zahran, Y. Abdin, and H. Salama, ARKIVOC, 11, 256–265 (2008).
B. Das, R. Pal, J. Banerjee, et al., Indian J. Chem. B., 44, 327–730 (2005).
S. S. Sonar, S. A. Sadaphal, A. H. Kategaonkar, et al., Bull. Korean Chem. Soc.,30, 825–828 (2009).
F. Shirini, M. Safarpoor Langroodi and M. Abedini, Chin. Chem. Lett.,21, 1342 – 1345 (2010).
N. O. Mahmoodi, M. Mohammadi Zeydi, and E. Biazar, J. Sulfur. Chem.,37, 613 – 621 (2016).
S. Handy and N. M. Westbrook, Tetrahedron Lett.,55, 4969 – 4971 (2014).
H. Veisi, R. Gholbedaghi, J. Malakootikhah, et al., J. Heterocyclic. Chem., 47, 1398 – 1305 (2010).
M. H. Sarvari, Acta. Chim. Slov.,54, 354 – 359 (2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soltani, S., Montazeri, N., Zeydi, M.M. et al. Synthesis of New Bis(Indolyl)Methanes Catalyzed by Benzylsulfamic Acid and Evaluation of Their Antimicrobial Activities. Pharm Chem J 53, 947–952 (2020). https://doi.org/10.1007/s11094-020-02103-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02103-3