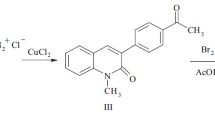
Reactions of 4-(4-bromoacetylphenyl)-3-hydroxy-2H-chromen-2-one with Py, 4-methylpyridine, quinoline, and benzo[f]quinoline produced quaternary salts; with dinucleophiles, derivatives of thiazole, imidazo[1,2-a]pyridine, and imidazo[1,2-a]pyrimidine. Several of the target compounds exhibited high antimicrobial activity that indicated further research on these compounds is warranted.
Similar content being viewed by others
References
L. Verotta, E. Lovaglio, G. Vidari, et al., Phytochemistry, 65, 2867 – 2879 (2004).
C. Billard, F. Menasria, C. Quiney, et al., Exp. Hematol., 36, 1625 – 1633 (2008).
C. Rappl, P. Barbier, V. Bourgarel-Rey, et al., Biochemistry, 45(30), 9210 – 9218 (2006).
R. Argotte-Ramos, G. Ramirez-Avila, M. C. Rodriguez-Gutierrez, et al., J. Nat. Prod., 69, 1442 – 1444 (2006); doi https://doi.org/10.1021/np060233p.
A. M. Zhyvoloup, S. M. Yarmoluk, M. M. Garazd, et al., Biopolym. Cell, 14(6), 553 – 558 (1998); http: //www.biopolymers.org.ua/pdf/uk/14/6/553/biopolym.cell-1998-14-6-553-uk.pdf.
J. Crecente-Campo, M. P. Vazquez-Tato, and J. A. Seijas, Eur. J. Org. Chem., 21, 4130 – 4135 (2010).
S. Combes, P. Barbier, S. Douillard, et al., J. Med. Chem., 54, 3153 – 3162 (2011).
J.-T. Pierson, A. Dumetre, S. Hutter, et al., Eur. J. Med. Chem., 45, 864 – 869 (2010).
C. Bailly, C. Bal, P. Barbier, et al., J. Med. Chem., 46(25), 5437 – 5444 (2003).
T. Kawate, N. Iwase, M. Shimizu, et al., Bioorg. Med. Chem. Lett., 23(22), 6052 – 6059 (2013).
G. Feuer, Prog. Med. Chem., 10, 85 – 158 (1974).
M. Khoobi, M. Alipour, S. Zarei, et al., Chem. Commun., 48, 2985 – 2987 (2012).
I.-T. Hwang, S.-A. Lee, J.-S. Hwang, and K.-I. Lee, Molecules, 16, 6313 – 6321 (2011).
M. M. Garazd, Ya. L. Garazd, and V. P. Khilya, Chem. Nat. Compd., 41(3), 245 – 271 (2005).
Z. Karimi-Jaberi and L. Zarei, Acta Chim. Slov., 60, 178 – 183 (2013).
H. R. Shaterian and M. Aghakhanizadeh, Chin. J. Catal., 34, 1690 – 1696 (2013).
B. Karami, S. Khodabakhshi, and K. Eskandari, Tetrahedron Lett., 53, 1445 – 1446 (2012).
B. C. Raju, T. H. Babu, and J. M. Rao, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 48, 120 – 123 (2009).
D. M. X. Donnelly, J.-P. Finet, P. J. Guiry, et al., Synth. Commun., 29(15), 2719 – 2730 (1999).
G. M. Boland, D. M. X. Donnelly, J.-P. Finet, et al., J. Chem. Soc., Perkin Trans. 1, 2591 – 2597 (1996).
L. Schio, F. Chatreaux, and M. Klich, Tetrahedron Lett., 41, 1543 – 1547 (2000).
A. Yu. Fedorov, A. V. Nyuchev, and I. P. Beletskaya, Khim. Geterotsikl. Soedin., 48(1), 175 – 186 (2012); A. Yu. Fedorov, A. V. Nyuchev, and I. P. Beletskaya, Chem. Heterocycl. Compd., 48(1), 166 – 178 (2012).
O. V. Skripskaya, N. O. Feilo, A. O. Neshchadin, et al., Zh. Org. Khim., 49(11), 1673 – 1678 (2013); O. V. Skripskaya, N. O. Feilo, A. O. Neshchadin, et al., Russ. J. Org. Chem., 49(11), 1655 – 1660 (2013).
O. V. Elenich, R. Z. Lytvyn, O. V. Blinder, et al., Khim.-farm. Zh., 52(12), 7 – 11 (2018); O. V. Elenich, R. Z. Lytvyn, O. V. Blinder, et al., Pharm. Chem. J., 52(12), 969 – 974 (2018).
D. P. Becker, D. L. Flynn, et al., US Pat. 5,260,303, Nov. 1, 1993; Chem. Abstr., 118, 254908n (1993).
J. J. Kaminski, C. Puchalski, D. M. Solomon, et al., J. Med. Chem., 32, 1686 – 1700 (1989).
K. Srimanth, V. R. Rao, and D. R. Krishna, Arzneim. Forsch., 52, 388 – 392 (2002).
I. V. Orlenko, S. N. Kovalenko, I. A. Zhuravel’, et al., Fiziol. Akt. Rechovini, 32(2), 25 – 28 (2001); I. V. Orlenko, S. M. Kovalenko, I. O. Zhuravel, et al., Physiol. Act. Subst., 32(2), 25 – 28 (2001).
Guide for Experimental (Preclinical) Studies of New Drugs [in Russian], Biont, Moscow, 2000, pp. 264 – 273.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 53, No. 9, pp. 20 – 25, September, 2019.
Rights and permissions
About this article
Cite this article
Rusnak, O.V., Lytvyn, R.Z., Skripskaya, O.V. et al. Synthesis and Antimicrobial Activity of 4-(4-Acetylphenyl)-3-Hydroxy-2H-Chromen-2-One Derivatives. Pharm Chem J 53, 797–802 (2019). https://doi.org/10.1007/s11094-019-02082-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02082-0