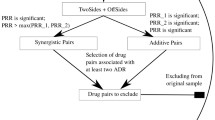
A structure–activity relationship model was constructed to predict adverse drug effects on the cardiovascular system. Models were constructed using sets of drug structures compiled by us with information about adverse effects from analyses and data integrated from various sources. The five most common cardiovascular adverse effects, i.e., myocardial infarction, ischemic stroke, cardiac failure, ventricular tachycardia, and arterial hypertension were analyzed in the work. The PASS software that has proved to be efficient in numerous studies was used to construct the appropriate models. The obtained models were accurate enough to be useful for estimating cardiovascular adverse effects of new drugs. An appropriate evaluation could be made in the very early stages of drug discovery because only the structural formula was needed for the prediction.


Similar content being viewed by others
References
J. J. Hornberg, M. Laursen, N. Brenden, et al., Drug Discovery Today, 19(8), 1131 – 1136 (2014).
T. A. Guskova, Khim.-farm. Zh., 35, No. 10, 3 – 8 (2001); Pharm. Chem. J., 35(10), 527 – 532 (2001).
S. M. Ivanov, A. A. Lagunin, and V. V. Poroikov, Drug Discovery Today, 21(1), 58 – 71 (2016).
O. A. Raevskii, A. N. Razdol’skii, Ya. V. Liplavskii, et al., Khim.-farm. Zh., 46(2), 3 – 8 (2012); Pharm. Chem. J., 46(2), 69 – 74 (2012).
M. Chen, V. Vijay, Q. Shi, et al., Drug Discovery Today, 16(15 – 16), 697 – 703 (2011).
M. Chen, A. Suzuki, S. Thakkar, et al., Drug Discovery Today, 21(4), 648 – 653 (2016).
S. M. Ivanov, A. A. Lagunin, A. V. Rudik, et al., J. Chem. Inf. Model., 58(1), 8 – 11 (2018).
E. J. Matthews and A. A. Frid, Regul. Toxicol. Pharmacol., 56(3), 247 – 275 (2010).
N. P. Tatonetti, P. P. Ye, R. Daneshjou, and R. B. Altman, Sci. Transl. Med., 4(125), 125ra31 (2012).
G. Jiang, H. Liu, H. R. Solbrig, and C. G. Chute, AMIA Jt. Summits Transl. Sci. Proc., 2013, 100 – 104 (2013).
M. Kuhn, I. Letunic, L. J. Jensen, and P. Bork, Nucleic Acids Res., 44(D1), D1075 – D1079 (2016).
J. M. Banda, L. Evans, R. S. Vanguri, et al., Sci. Data, No. 3, 160026 (2016).
URL: http://informatics.mayo.edu/adepedia/index.php/MainPage
A. P. Davis, C. J. Grondin, R. J. Johnson, et al., Nucleic Acids Res., 45(D1), D972-D978 (2017).
URL: http://ctdbase.org/
D. Fourches, E. Muratov, and A. Tropsha, Nat. Chem. Biol., 11(8), 535 (2015).
D. A. Filimonov and V. V. Poroikov, Ross. Khim. Zh., 50(2), 66 – 75 (2006).
D. A. Filimonov, A. A. Lagunin, T. A. Gloriozova, et al., Khim. Geterotsikl. Soedin., No. 3, 483 – 499 (2014); Chem. Heterocycl. Compd., 50(3), 444 – 457 (2014).
V. M. Bezhentsev, D. S. Druzhikovskii, S. M. Ivanov, et al., Khim.-farm. Zh., 51(2), 3 – 11 (2017); Pharm. Chem. J., 51(2), 91 – 99 (2017).
D. S. Druzhikovskii, A. V. Rudik, D. A. Filimonov, et al., Izv. Akad. Nauk, Ser. Khim., No. 10, 1832 – 1841 (2017); Russ. Chem. Bull., Int. Ed., 66(10), 1832 – 1841 (2017).
P. M. Vasil’ev, K. Yu. Kalitin, A. A. Spasov, et al., Khim.-farm. Zh., 50(12), 3 – 8 (2016); Pharm. Chem. J., 50(12), 775 – 780 (2016).
V. I. Zvarich, M. V. Stasevich, O. V. Stan’ko, et al., Khim.-farm. Zh., 48(9), 20 – 24 (2014); Pharm. Chem. J., 48(9), 584 – 588 (2014).
S. A. Kryzhanovskii, R. M. Salimov, A. A. Lagunin, et al., Khim.-farm. Zh., 45(10), 25 – 31 (2011); Pharm. Chem. J., 45(10), 605 – 611 (2012).
K. A. Murtazalieva, D. S. Druzhilovskiy, R. K. Goel, et al., SAR QSAR Environ. Res., 28(10), 843 – 862 (2017).
D. Filimonov and V. Poroikov, in: Chemoinformatics Approaches to Virtual Screening, A. Varnek and A. Tropsha (eds.), RSC Publishing, Cambridge (2008), pp. 182 – 216.
B. Sibbald, CMAJ, 171(9), 1027 – 1028 (2004).
A. C. Pessina, M. Hlede, F. Morandin, et al., Int. J. Clin. Pharmacol. Res., 3(1), 41 – 55 (1983).
Acknowledgments
The work was sponsored by the Russian Science Foundation (Project No. 17 – 75 – 10168).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 9, pp. 8 – 13, September, 2018.
Rights and permissions
About this article
Cite this article
Ivanov, S.M., Lagunin, A.A., Filimonov, D.A. et al. Computer Prediction of Adverse Drug Effects on the Cardiovascular System. Pharm Chem J 52, 758–762 (2018). https://doi.org/10.1007/s11094-018-1895-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1895-1