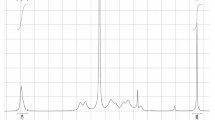
The first step of low-molecular-mass heparin (enoxaparin) preparation by hydrolytic depolymerization of unfractionated heparin was investigated. The step involved chemical reaction of starting heparin and benzethonium chloride in aqueous saline solutions. The number of acid equivalents in unfractionated heparin was shown to increase as the NaCl concentration increased. This was probably related to effects of the solution ionic strength on the heparin macromolecular conformation, which was confirmed using dynamic light scattering. The average size of the observed light-scattering centers in aqueous heparin solutions decreased with increasing NaCl concentration. Rinsing with H2O and not NaCl solutions and application of ultrasonication were recommended to accelerate purification of benzethonium heparinate from starting materials and to reduce the amount of rinse water. The compositions of the produced and purified benzethonium heparinate samples were confirmed using PMR spectroscopy.










Similar content being viewed by others
References
B. P. Lukashin, A. N. Grebenyuk, and V. V. Zatsepin, Vestn. Ross. Voenno-Med. Akad., 20(4), 141 – 147 (2007).
A. L. Berkovskii, E. V. Sergeeva, A. V. Suvorov, et al., Methods for Determining Heparin Activity [in Russian], GBOU DPO RMAPO, Moscow (2015), pp. 10 – 62.
E. A. Kurakina, Fundam. Issled., No. 4, 69 – 73 (2012).
E. N. Kondrashenko, A. V. Butrov, and A. B. But-Gusaim, Trudnyi Patsient, 10(11), 33 – 36 (2012).
I. S. Yavelov, Klin. Farmakol. Ter., 19(1), 56 – 63 (2010).
L. E. Frumin, G. A. Kostakova, et al., RU Pat. No. 2,512,768, Apr. 10, 2014.
E. M. Shulutko, Trudnyi Patsient, 9, No. 4, 42 – 49 (2011).
M. N. Kudykin, Statsionarozameshch. Tekhnol.: Ambul. Khir., No. 3 – 4 (59 – 60), 35 – 41 (2015).
Z. Liu, S. Ji, J. Sheng, and F.Wang, Drug Discovery Ther., 8(1), 1 – 10 (2014).
R. Linhardt and N. Gunay, Semin. Thromb. Hemost., 25(3), 5 – 25 (1999).
O. L. Romanova and N. V. Sturov, Trudnyi Patsient, 9(10), 32 – 36 (2011).
S. A. Golubev, Nov. Khir., 15(3), 83 – 90 (2007).
A. A. Gadel'shina, Int. J. Appl. Fundam. Res., No. 9, 354 – 356 (2016).
P. Bianchini, L. Liverani, et al., EP Pat. No. 1,510,528, Mar. 2, 2005.
Acknowledgments
The work was financially supported by the Ministry of Education and Science of the Russian Federation under the project part of a state task for 2017 – 2019 (Project 15.2463.2017/4.6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frumin, L.E., Yur’eva, K.P., Askretkov, A.D. et al. Optimization of the First Step of Enoxaparin Synthesis by Hydrolytic Depolymerization of Unfractionated Heparin. Pharm Chem J 52, 735–739 (2018). https://doi.org/10.1007/s11094-018-1893-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1893-3