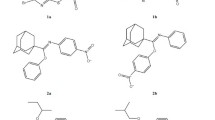
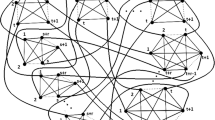
A database of some S,N,N,N′-tetrasubstituted isothiourea derivatives possessing neuroprotective properties was successfully clustered in order to study the quantitative structure – activity relationship. Clustering by k-means was carried out in the factor space of topological descriptors. The identified clusters were combined according to analyses of intra- and intercluster distances. The initial number of clusters in the k-means clustering was determined from the number of iterations for which a solution was obtained. The homogeneity of the database and the identified clusters was estimated by using a coefficient of molecular diversity. A plot of the database compounds as points in factor space led to a conclusion about the successful applicability of the proposed clustering approach.





Similar content being viewed by others
References
D. K. Agrafiotis, D. Bandyopadhyay, J. K. Wegner, and H. van Vlijmen, J. Chem. Inf. Model., 47, 1279 – 1293 (2007).
R. Guha and P. C. Jurs, J. Chem. Inf. Model., 45, 800 – 806 (2005).
D. M. Hawkins, S. C. Basak, and X. Shi, J. Chem. Inf. Comput. Sci., 41, 663 – 670 (2001).
I. I. Baskin, V. A. Palyulin, and N. S. Zefirov, J. Chem. Inf. Comput. Sci., 37(4), 715 – 721 (1997).
M. Kumar, K. Thurow, N. Stoll, and R. Stoll, Eur. J. Med. Chem., 42, 675 – 685 (2007).
G. Downs and J. Barnard, in: Reviews in Computational Chemistry, Vol. 18, K. Lipkowitz and D. Boyd (eds.), Wiley-VCH, New York (2002), pp. 1 – 40.
T. Varin, R. Bureau, C. Mueller, and P.Willett, J. Mol. Graphics Modell., 28(2), 187 – 195 (2009).
G. J. Niemi, in: Practical Applications of Quantitative Structure – Activity Relationships (QSAR) in Environmental Chemistry and Toxicology, W. Karcher and J. Devillers (eds.), Kluwer Academic Publishers, Dordrecht, Boston, London (1990), pp. 153 – 169.
A. Smellie, J. Chem. Inf. Comput. Sci., 44, 1929 – 1935 (2004).
S. V. Trepalin and A. V. Yarkov, J. Chem. Inf. Comput. Sci., 41, 100 – 107 (2001).
G. L. Perlovich, A. N. Proshin, T. V. Volkova, et al., J. Med. Chem., 2(7), 1845 – 1852 (2009).
S. Bachurin, I. Baskin, V. Grigoriev, et al., Drugs Future, 29, Supp. A, 188 (2004).
R. Todeschini and V. Consonni, Handbook of Molecular Descriptors, Wiley-VCH Publishers, Weinheim (2000).
J. D. Holliday, S. L. Rodgers, and P. Willett, J. Chem. Inf. Comput. Sci., 44, 894 – 902 (2004).
R. Stanforth, E. Kolossov, and B. Mirkin, in: Selected Contributions in Data Analysis and Classification, P. Brito, P. Bertrand, G. Cucumel, and F. de Carvalho (eds.), Springer, (2007), Part II, pp. 225 – 233.
Acknowledgments
The work was performed with support from the Russian Science Foundation Project No. 14 – 23 – 00160. Equipment of the Center for Collective Use, IPAC, RAS (contract No. 14.621.21.0008, identifier REMEFI62114X0008) was used in the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 51, No. 4, pp. 20 – 29, April, 2017.
Rights and permissions
About this article
Cite this article
Andreeva, E.P., Proshin, A.N., Serkov, I.V. et al. Application of Molecular Topological Descriptors for Clustering a Database of Isothiourea Derivatives in Studying Structure – Activity Relationships. Pharm Chem J 51, 262–271 (2017). https://doi.org/10.1007/s11094-017-1595-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-017-1595-2