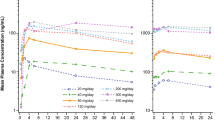
A selective and specific quantitative assay with HPLC/MS determination of the broad-spectrum antituberculosis antibiotic cycloserine in blood plasma with reserpine internal standard was developed and validated. It was shown that the method was accurate and precise and linear in the range 0.5 – 20.0 μg/mL. A calibration curve obeyed the equation Y = aX + b, where a = 0.0893 and b = –0.0134 with a correlation coefficient of 0.9994. The cycloserine limit of detection was 0.1 μg/mL; the limit of quantitation, 0.5 μg/mL. The recoveries of cycloserine from the matrix at low (0.5 μg/mL) and high (20.0 μg/mL) drug concentrations were 41.7 and 62.4%, respectively, with matrix factors of 0.28 and 0.34, respectively, normalized to the internal standard.



Similar content being viewed by others
References
Guide to Drugs in the Drug Registry: Instructions, Use, and Description. Cycloserine [in Russian], http://www.rlsnet.ru/mnnindexid1416.htm
V. David, M. Ionescu, and V. Dumitrescu, J. Chromatogr. B: Biomed. Sci. Appl., 761, 27 – 33 (2001).
J. J. Pesek, M. T. Matyska, and A. Dang, J. Pharm. Biomed. Anal., 64 – 65, 72 – 76 (2012).
S. Polagani, N. Pilli, R. Maddela, et al., J. Pharm. Biomed. Anal., 76, 21 – 27 (2013).
Guideline on Bioanalytical Method Validation, Eur. Med. Agency (2011).
Acknowledgments
The work used equipment at the Shared Research and Educational Center, PFUR.
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 50, No. 2, pp. 42 – 46, February, 2016.
Rights and permissions
About this article
Cite this article
Stepanova, E.S., Ovcharov, M.V., Barsegyan, S.S. et al. Determination of Cycloserine in Blood Plasma by HPLC/MS: Application to Bioequivalence Studies. Pharm Chem J 50, 195–199 (2016). https://doi.org/10.1007/s11094-016-1422-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1422-1