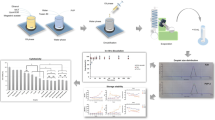
Microcrystals of 6α-methyl-16α,17α-cyclohexapregn-4-ene-3,20-dione (mecigestone) that differed from the starting drug substance by decreased sizes and altered morphologies were produced using a Nano Spray Dryer B-90 (Buchi, Switzerland) that allowed operation with organic solvents in a closed cycle. The bioavailability of starting crystalline and micronized mecigestone drug substance was studied by oral administration to laboratory white rats. Mecigestone was extracted from rat blood serum using solvent extraction. The extraction coefficient was 64%. The detection limit of the drug substance was 0.08 ng/mL. The content of unaltered mecigestone in the extracts was determined using HPLC with high-resolution mass spectrometric detection. Micronized mecigestone was 7.5 times more bioavailable than the starting drug substance.





Similar content being viewed by others
References
K. P. Madauss, E. L. Stewart, and S. P. Williams, Med. Res. Rev., 27, 374 – 400 (2007).
A. V. Kamernitskii and I. S. Levina, Bioorg. Khim., 31, 115 – 129 (2005).
A. V. Kamernitskii and I. S. Levina, Bioorg. Khim., 31, 227 – 238 (2005).
N. L. Shimanovskii, A. V. Semeikin, T. A. Fedotcheva, et al., Byull. Eksp. Biol. Med., No. 10, 447 – 450 (2002).
S. V. Emshanova, Khim.-farm. Zh., 42(2), 38 – 43 (2008); Pharm. Chem. J., 42(2), 89 – 94 (2008).
N. V. Efremova and I. N. Topchieva, Biokhimiya, 58(7), 1071 – 1076 (1993).
J. L. Benifia, M. Dumont, M. Levardion, et al., Contracept. Fertil. Sex., 25, 165 – 169 (1997).
N. Rasenack, H. Streckel, and B. W. Muller, J. Pharm. Sci., 92(1), 35 – 44 (2003).
Y. P. Sun, M. J. Meziani, P. Pathak, et al., Chem. Eur. J., 11(5), 1366 – 1373 (2005).
K. Moribe, S. Tsutsumi, S. Morishita, et al., Chem. Pharm. Bull., 53(8), 1025 – 1028 (2005).
N. Schafroth, C. Arpagaus, U. Y. Jadhav, et al. Colloids Surf., B, 90, 8 – 15 (2012).
A. S. Kashin and V. P. Ananikov, Izv. Akad. Nauk, Ser. Khim., No. 12, 2551 – 2556 (2011).
Acknowledgments
We thank the Division of Structural Research, Inst. Org. Chem., RAS, for performing the electron microscope studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 49, No. 10, pp. 44 – 48, October, 2015.
Rights and permissions
About this article
Cite this article
Nazarov, A.K., Zavarzin, I.V., Nazarov, G.V. et al. Preparation and Bioavailability Evaluation of Micronized Steroidal Mecigestone Drug Substance. Pharm Chem J 49, 706–710 (2016). https://doi.org/10.1007/s11094-016-1357-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1357-6