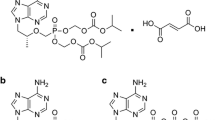
Hepatitis B virus (HBV) is a global threat that killed many human lives. HBV DNA polymerase (HDP) is the key target for antiviral drug treatment. The widely used drug against HBV infection is lamivudine which targets the reverse transcriptase activity of HDP and inhibits the replication of HBV. However, available evidence demonstrated that tyrosine (Y)-methionine (M)-aspartic acid (D)-aspartic acid (D) motif mutations significantly affected the efficacy of lamivudine binding. In particular, M204I mutations affect the drug binding mechanism and cause resistance to lamivudine. Therefore, in the present study we made an attempt to understand the mechanism of lamivudine resistance with the aid of molecular docking and molecular dynamics (MD) approach. The molecular docking results suggest that lamivudine adopts the most promising conformations to the native type HDP by identifying M-204 and Y-203 as a prospective partner for making polar contacts as compared to the mutant type HDP. The MD results showed that the average movements of atoms, especially atoms of the native type HDP– lamivudine complex, were small and displayed fast convergence of energy and charges in geometry. This highlights the stable binding of lamivudine with native type HDP as compared to mutant type HDP. The R2 and RMSF analysis certainly indicates conformational changes in the HDP structure due to M204I mutation. Furthermore the hydrogen bond (H-bond) analysis from the MD study showed that there is decreased number of intermolecular H-bonds in mutant HDP – lamivudine complex as compared to that in native type HDP – lamivudine complex. Overall, our study certainly will pave way to develop new drugs against the drug resistant mutations (M204I) of HBV.






Similar content being viewed by others
References
T. J. Liang, Hepatology, 49, 13 – 21 (2009).
D. Lavanchy, J. Clin. Virol., 34, 1 – 3 (2005).
S. A. Wynne, R. A. Crowther, and A. G. Leslie, Mol. Cell, 6, 771 – 780 (1999).
B. J. McMahon, Hepatol. Intern., 3, 334 – 342 (2009)
C. Mayerat, A. Mantegani, and P. C. Frei, J. Viral Hepatol., 6, 299 – 304 (1999).
K.-H. Kim, N. D. Kim, and B.-L. Seong, Molecules, 15(9), 5878 – 5908 (2010).
M. Seifer and D. N. Standring, J. Virol., 67, 4513 – 4520 (1993).
L. Lin and J. Hu, J. Virol., 82(5), 2305 – 2312 (2008).
F. Zoulim, Liver Intern., 1, 111 – 116 (2011).
M. Seifer, A. Patty, I. Serra, et al., Antivir. Res., 81(2), 147 – 155 (2009).
T. T. Chang, R. G. Gish, R. de Man, et al., New Engl. J. Med., 354(10), 1001 – 10 (2006).
B. Dikici, M. Bosnak, I. H. Kara, et al., Pediatr. Infect. Dis. J., 20(10), 988 – 992 (2001).
M. F. Yuen and C. L. Lai, J. Antimicrob. Chemother., 51(3), 481 – 485 (2003).
R. K. Gaillard, J. Barnard, V. Lopez, et al., Antimicrob. Agents Chemother., 46 (4), 1005 – 1013 (2002).
T. Schwede, J. Kopp, N. Guex, and M. C. Peitsch, Nucl. Acid Res., 31(13), 3381 – 3385 (2003).
N. Guex and M. C. Peitsch, Electrophoresis, 18(15), 2714 – 2723 (1997).
B. Hess, C. Kutzner, D. Spoel, and E. Lindahl, J. Chem. Theory Comput., 4, 435 – 447 (2008).
D. Spoel, E. Lindahl, B. Hess, et al., J. Comput. Chem., 26(16), 1701 – 1718 (2005).
J. Feldman, K. A. Snyder, A. Ticoll, et al., FEBS Lett., 580, 1649 – 1653 (2006).
J. Gasteiger, C. Rudolph and J. Sadowski, Tetrahedron Comput Meth., 3, 537 – 547. (1990).
P. R. Daga, J. Duan, and R. J. Doerksen, Protein Sci., 19, 796 – 807 (2010).
A. M. Ismail, O. P. Sharma, M. S. Kumar, et al., Bioinformation, 9, 121 – 125 (2013).
A. W. Walsh, D. R. Langley, R. J. Colonno, et al., PLoS One, 5, e9195 (2010).
R. A. Laskowski, M. W. MacArthur, D. S. Moss, and J. M. Thornton, J. Appl. Cryst., 26(2), 283 – 2911 (1993).
Z. Yuan, T. L. Bailey, and R. D. Teasdale, Proteins, 58, 905 – 912 (2005).
D. Ringe and G. A. Petsko, Methods Enzymol., 131, 389 – 433 (1986).
S. Parthasarathy and M. R. Murthy, Protein Eng., 13, 9 – 13 (2000).
H. A. Carlson and J. A. McCammon, Mol. Pharmacol., 57, 213 – 218 (2000).
V. Karthick, V. Shanthi, R. Rajasekaran and K. Ramanathan, Protoplasma, 250(1), 197 – 207 (2013).
R. Rajasekaran, C. George Priya Doss, C. Sudandiradoss, et al., C. R. Biol., 331, 409 – 417 (2008).
A. Hinkle and L. S. Tobacman, J. Biol. Chem., 278, 506 – 513 (2003).
K. Suhre and Y. H. Sanejouand, Nucl. Acids Res., 32, 610 – 614 (2004).
A. Oda, M. Okayasu, Y. Kamiyama, et al., Bull. Chem. Soc. Jpn., 80, 1920 – 1925 (2007).
A. C. Wallace, R. A. Laskowski, and J. M. Thornton, Protein Eng., 8, 127 – 134 (1995).
K. L. Meagher and H. A. Carlson, Proteins, 58, 119 – 125 (2005).
A. W. Schuttelkopf and D. M. F. Van Aalten, Acta Crystallogr., 60, 1355 – 1363 (2004).
T. Darden, L. Perera and L. Pedersen, Structure, 7, 55 – 60 (1999).
E. Hindahl, B. Hess and D. van der Spoel, J. Mol. Model., 7, 306 – 317 (2001).
R. T. Kroemer, Curr. Protein Pept. Sci., 8, 312 – 328 (2007)
J. Fung, C. L. Lai, W. K. Seto, and M. F. Yuen, J. Antimicrob Chemother., 66, 2715 – 2725 (2011).
Acknowledgments
The authors express deep gratitude to management of the Vellore Institute of Technology for all the support, assistance, and constant encouragement to carry out this work
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srividhya, M., Ramanathan, K. Molecular Dynamics Simulation Approach to Understand Lamivudine Resistance in Hepatitis B Virus Polymerase. Pharm Chem J 49, 432–438 (2015). https://doi.org/10.1007/s11094-015-1300-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1300-2