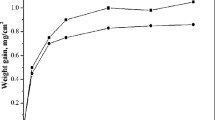
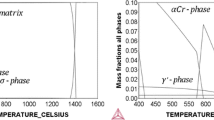
Abstract
In previous work, it was shown that CaSO4-induced corrosion can cause significant internal oxidation in single-crystal Ni-based superalloys similar to that observed in field-exposed components when subjected to a bi-thermal exposure (8 h at 1150 °C → 60 h at 871 °C) in air + 30% steam. In this work, the influence of (1) 1150 °C initiation stage temperature, (2) propagation stage temperature, and (3) CaSO4 deposit mass on the mechanism of CaSO4-induced corrosion were investigated further. The results of these experiments showed that subsurface depletion of Al and Cr caused by rapid Ca4Al6O16S, CaAl2O4, and CrS formation due to CaSO4 corrosion is required for internal oxidation to occur in N5 and N500, but that these alloys can “heal” when held for long durations at 1150 °C due to the enhanced diffusion of Al within the alloy at elevated temperatures.













Similar content being viewed by others
References
T. J. Nijdam and R. van Gestel, Natl. Lucht- en Ruimtevaartlaboratorium, no. NLR-TP-2011-547, (2010)
M. B. Krisak, B. I. Bentley, A. W. Phelps, and T. C. Radsick, Journal of Propulsion and Power 33, (3), 2017 (697–703).
P. T. Brennan, D. Konitzer, M. Brennan, and B. Gleeson, Oxidation of Metals 98, 2022, (43–63).
C. S. Giggins and F. S. Pettit, Journal of the Electrochemical Society 118, (11), 1971 (1782–1790).
A. Durga, H. Dai, S. Huang, I. Spinelli, and L. Yuan, JOM 72, 2020 (1785–1793).
T. Gheno, G. H. Meier, and B. Gleeson, Oxidation of Metals 84, 2015 (185–209).
Thermo-Calc Software TCNI Database.
B. Gleeson, Materials Science and Technology: A Comprehensive Treatment: Corrosion and Environmental Degradation. (2000). pp. 173–228.
C. J. Spengler and R. Viswanathan, Metallurgical and Materials Transactions B 3, 1972 (161–166).
J. L. Meijering, Advances in Materials Science. Wiley, 1971.
R. A. Rapp, Corrosion 21, (12), 1965 (382–401).
J. W. Park and C. J. Altstetter, Metallurgical Transactions A 18, (1), 1987 (43–50).
M. S. A. Karunaratne, P. Carter, and R. C. Reed, Acta Materialia 49, (5), 2001 (861–875).
P. Carter, B. Gleeson, and D. J. Young, Acta Materialia 44, (10), 1996 (4033–4038).
Acknowledgements
Funding support of this study by the Office of Naval Research (Grant N00014-17-1-2916), Dr. David Shifler. Program Manager is greatly appreciated.
Author information
Authors and Affiliations
Contributions
PB completed the research and wrote the manuscript. DK and MB assisted me during the research process by providing access to field exposed components, test coupons, and expertise relating to the corrosion in gas turbine engines (both worked at GE Aviation at the time the research was conducted). BG was my PhD advisor.
Corresponding author
Ethics declarations
Conflict of interest
I declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The following outlines the steps used to quantify the Al-consumption rate of CaSO4-induced degradation relative to that due to Al2O3-scale formation at 1150 °C. The solution used is based on work from [14]. Using the boundary conditions given in Fig. 12 and the measured denuded zone depth of 40.3 μm after 86400 s (24 h), the growth of the γ’ denuded zone can be modeled by Eq. 1 as presented by Carter et al. [14], i.e.,
where \({N}_{Al}^{\gamma }\) is the Al concentration at the γ − γ + γ’ interface (9.9 at%), \({N}_{Al}^{o}\) is the Al concentration in the bulk alloy (13.9 at%), and \({N}_{Al}^{i}\) is the Al concentration at the scale-alloy interface (7.0 at%).
The quantity α from the standard solution is given in Eq. 2, with α = 0.354 when solved using Xd = 4.03 × 10–3 cm for the γ’ depletion zone depth, \({D}_{Al}^{Ni}\) = 3.746 × 10–10 \(\frac{{cm}^{2}}{s}\) at 1150 °C (based on data from [13]), and t = 86400 s.
Substituting α = 0.354 into Eq. 1 and simplifying yields erf(u) = − 0.636, which corresponds to u = − 0.642. The u in Eq. 1 is given by Eq. 3, where kc \(\left(\frac{{cm}^{2}}{s}\right)\) is the corrosion constant that represents the Al consumption rate in terms of equivalent Al metal recession and \({D}_{Al}^{Ni}\) = 3.5 × 10–10 \(\frac{{cm}^{2}}{s}\) at 1150 °C. Solving yields kc = 6.1 × 10–10 \(\frac{{cm}^{2}}{s}\)
This kc can be converted to the equivalent growth-rate constant of Al2O3 that would form a γ’ denuded zone that is 4.03 × 10–3 cm thick in 86400 s using Eq. 4. This rate constant is \({k}_{p}^{DZ}\) = 3.5 × 10–3 \(\frac{{mg}^{2}}{{cm}^{4}s}\).
Thus, the equivalent Al2O3 growth-rate constant deduced from the γ’-denuded-zone depth in CaSO4-deposited alloys is roughly 1000–4000 times greater than that for typical Al2O3-scale growth.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brennan, P.T., Konitzer, D., Brennan, M. et al. Environmental Factors Affecting the CaSO4-Induced Corrosion of Ni-Based Superalloys N5 and N500. High Temperature Corrosion of mater. 101, 149–168 (2024). https://doi.org/10.1007/s11085-023-10193-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-023-10193-z