Abstract
Prebiotic processes required a reliable source of free energy and complex chemical mixtures that may have included sugars. The formose reaction is a potential source of those sugars. At moderate to elevated temperature and pH ranges, these sugars rapidly decay. Here it is shown that CaCO3-based chemical gardens catalyze the formose reaction to produce glucose, ribose, and other monosaccharides. These thin inorganic membranes are explored as analogs of hydrothermal vent materials—a possible place for the origin of life—and similarly exposed to very steep pH gradients. Supported by simulations of a simple reaction-diffusion model, this study shows that such gradients allow for the dynamic accumulation of sugars in specific layers of the thin membrane, effectively protecting formose sugar yields. Therefore, the formose reaction may be a plausible prebiotic reaction in alkaline hydrothermal vent environments, possibly setting the stage for an RNA world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A particularly interesting setting to consider for the origins of life are hydrothermal vents, both here on Earth and among some of the icy moons of the outer solar system (Brandscom et al. 2017, Ruiz-Mirazo et al. 2014; Martin et al. 2008; Martin and Russell 2007; Russell et al. 2014). These geologically diverse environments are energetically and minerally rich, and exhibit pH and thermal gradients that could be of great use to prebiotic chemistry (Martin and Russell 2007; Russell et al. 2014; Möller et al. 2017). Among the chemical species needed for the emergence of life, especially under the hypothesis of an RNA world, sugars play an important part in life because they are essential for the formation of certain biopolymers and the control of energy flow (Horecker 1976; Orgel 2004). In this context, the formose reaction gained prominence because it reacts formaldehyde (CH2O) generating glycolaldehyde in a slow induction step and then in a much quicker phase produces a multitude of higher molecular weight sugars autocatalytically (Breslow 1959; Butlerow 1861). The formose reaction is catalyzed by calcium hydroxide classically, and other divalent metal ions as well (Breslow 1959; Butlerow 1861; Ricardo et al. 2004; Weiss et al. 1970). The underlying mechanism involves aldol reactions, reverse aldol reactions, and aldose-ketose isomerization while glycolaldehyde, glyceraldehyde, dihydroxyacetone, and tetrose sugars are important, early intermediates (Breslow 1959; Butlerow 1861; Ricardo et al. 2004; Weiss et al. 1970).
The formose reaction is geochemically plausible (Schwartz and De Graaf 1993). In the geological context of early Earth or other planets/moons with a rock-ocean interface, formaldehyde might have been delivered by meteorites. This hypothesis is supported by recent studies on the abundance of formaldehyde in the interstellar space, comets, and interplanetary dust particles (Cody et al. 2011). In addition, evidence exists for a photochemical and electrochemical production of formaldehyde and formate in the CO2 dominated atmosphere of the early Earth (Cody et al. 2011; Pinto et al. 1980; Wen et al. 1989; Löb 1906). Due to atmospheric, geologic and meteor inputs, formaldehyde may have been readily available to hydrothermal environments, and given concentrating mechanisms produced sugars. While some vents such as the immensely hot black smokers may be too hot for formaldehyde to remain stable (Cleaves 2008), temperate alkaline vents may have been viable still. These vents could have acted as concentrators for formaldehyde on the early Earth, like the ideas of hydrothermal sediments acting as concentrators (Westall et al. 2018). Past studies have established the different aspects of the formose reaction are plausibly geochemical, requiring concentration mechanisms, and not directly yielding appreciable amounts of a specific sugar directly (Schwartz and De Graaf 1993, Cleaves 2008).
The relevance of the formose reaction to prebiotic processes faces doubts due to the issue of sugar stability. A sugar stability study by Larralde et al. 1995 showed that sugars decompose under basic conditions and, at moderate to elevated temperatures, even for near neutral pH values. For example, ribose decomposes at pH 7.4 with a half-life of 73 min at 100 °C but is mostly stable at 25 °C. The criticism further cited browning (Ajandouz et al. 2008) reactions of sugars with amino acids that occur at temperatures above 140 °C and other decay channels such as the oxidation to sugar acids.
This study investigates the relevance of the formose reaction with an emphasis on the membrane-like features of hydrothermal vent structures and their flow-driven far-from-equilibrium conditions. Hydrothermal vent chimneys are naturally occurring examples of chemical gardens (Hughes et al. 2017). Chemical gardens are hollow tubes that form by the rapid precipitation of metal carbonates, phosphates, hydroxides, oxides and amorphous silica (Hughes et al. 2017).
This work utilized so-called chemical gardens as vent chimney material analogues and shows that calcium-based chemical gardens catalyze the formose reaction. Additionally, using a simple reaction-diffusion model, this study shows that the membrane-like walls of the chemical garden provide a complex environment for the production and accumulation of sugars in the presence of transmembrane gradients. These conditions would indicate the formose reaction may be plausible in the environments of alkaline hydrothermal vents.
Materials and Methods
Chemical Garden Synthesis via Pump Injection
Precipitation reactions took place in a rectangular reaction vessel made of plexiglass. The vessel had a base measuring 4 cm × 4 cm and a height of 10 cm. Inserted into the base is a plastic capillary (length 1 cm, inner diameter 0.61 mm) which connected to a syringe (HSW NORM-JECT, 20 mL) via flexible tubing. The syringe contained aqueous calcium chloride solution (CaCl2·2H2O, Fisher) at a concentration of 0.125 M or 0.25 M and is inserted into a programmable syringe pump (New Era Pump Systems Inc, NE-300), which delivers the calcium solution at a rate of 1.0 mL/min into a 1.0 M aqueous solution of sodium meta-silicate that also contains 100 mM Na2CO3 (Fisher). These syntheses were carried out at 25 °C.
Chemical Garden in Formaldehyde Solution Exposed to Hydrothermal Conditions
The calcium based chemical gardens (equivalent to a total length of about 5 cm, 0.05 g hydrated) were transferred into a 125 mL reaction flask and submerged in 100 mL solution containing 1 M formaldehyde (Macron) and 0.5 M methanol (Macron). The solid tube material is hydrated and has not been dried. The solution was titrated with sodium hydroxide to the desired pH. For hydrothermal synthesis of the formose sugars, the flask top is covered with aluminum foil and autoclaved for 1 hour at 120 °C and 200 kPa.
Some experiments required observation and sampling of the formose solutions in real time. For lower temperature heating conditions that are observable with the naked eye formaldehyde solutions were loaded into a hot water bath placed on a hotplate (Corning 6795 Stirring Hot Plate) in the fume hood. The temperature of the water bath was monitored with thermometers and controlled with an attached thermocouple sensor. Solutions were then heated for the determined time and temperature based on the experiment and observed for yellowing, the production of formose products was verified spectroscopically with NMR. After initial data were collected the experiments were run in triplicate with and without 100 mM glycolaldehyde (Sigma-Aldrich) added to solutions.
Characterization of Formose Reaction Products
Filtered product solutions were characterized using 1D proton magnetic resonance measurements with ZGGPW5 water suppression pulses on a Bruker 600 MHz NMR. 100 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS, Sigma-Aldrich) and 10% by volume D2O were used to standardize a reference point and lock spectral measurements.
In addition, glycosyl composition analysis was performed by combined gas chromatography/mass spectrometry (GC/MS) of the per-O-trimethylsilyl (TMS) derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis. The dried formose sample (1.8 mg) with inositol (internal standard, 20 µg) was heated with methanolic HCl in a sealed screw-top glass test tube for 18 h at 80 °C. After cooling and removal of the solvent under a stream of nitrogen, the samples were treated with a mixture of methanol, pyridine, and acetic anhydride for 30 minutes. The solvents were evaporated and the samples derivatized with Tri-Sil (Pierce) at 80 °C for 30 min. GC/MS analysis of the TMS methyl glycosides is performed on an Agilent 7890A GC interfaced to a 5975C MSD, used a Supelco Equity-1 fused silica capillary column (30 m × 0.25 mm ID). The mixture of derivatized sample was analyzed by GC-MS and per-O-trimethylsilyl derivatives of monosaccharide residues run alongside the sample and used for quantification of each monosaccharide residue. The peak integration was used for quantification as compared to each standard after calculating the response factor for each residue.
Microfluidic Experiments
Microfluidic devices were assembled to investigate pH changes across the inorganic precipitate membrane. They consisted of a cut parafilm membrane placed between two plexiglass plates (25 mm × 75 mm). In addition, two barb fittings were glued onto the upper plexiglass plate using an epoxy adhesive. A Y-shaped pattern was cut out from the parafilm as the channel for the reaction using an inexpensive electronic cutting tool (Silhouette Portrait) connected to a PC.
The straight part of the channel is 55 mm long, 3 mm wide, and approximately 0.13 mm high. The assembled device was heated on a hotplate at low heat until the parafilm membrane melts slightly and becomes transparent. Upon cooling, the solidified parafilm acted as an adhesive to secure the device. Once the construction of the microfluidic device was completed, two syringes containing Na2CO3 solution (0.4 M) and CaCl2 solution (0.4 M) are connected to the barb fittings with plastic tubing (Tygon, inner diameter 1/16”). Both syringes contained either saturated bromothymol blue or phenol red solution as a pH indicator and are simultaneously discharged into the microfluidic device at a rate of 1 mL/h per syringe using a programmable syringe pump (New Era Pump Systems, NE 1600). Prior to the experiment, the channel was filled with water. All microfluidic experiments are performed at room temperature. The formation of the precipitate membranes is observed under white light with an inverted microscope (Leica DM IRB) and recorded with a digital camera (Nikon D3300).
Model and Numerical Simulations
Taking into consideration the model, flow of incoming ocean water is considered the primary of the source of formaldehyde. Upwelling flow of hydrothermal fluid is considered the primary source for hydroxides. Temperature and pH gradients are assumed across the membranes of the hydrothermal chimney from the hot basic sediment to the cooler more acidic ocean.
This simple model considers the most fundamental reaction-diffusion processes within the membrane (width w) in terms of the concentrations of formaldehyde f(x,t) and a generic sugar species s(x,t), where x and t are the transmembrane distance and time, respectively. It assumes that there is neither formaldehyde nor sugar in the (interior) vent fluid (x ≥ w). It further assumes that the surrounding ocean (x < 0) is well-mixed, therefore, the ocean is sugar-free and forms a formaldehyde pool of constant concentration (here set to 1 without loss of generality).
Accordingly, the model employs the following general boundary conditions, at time zero formaldehyde concentration is present or equal to 1, also no sugar is present at time zero, as time proceeds formaldehyde concentration decreases and sugar concentration increases. As formaldehyde is reacted to form the sugars the formaldehyde concentration and the sugars rely on the membrane wall and its thickness for preservation ( f(0,t) = 1 and f(w,t) = s(0,t) = s(w,t) = 0 for all t). Notice that these assumptions are conservative as even a slight accumulation of sugars and formaldehyde (the latter for x > w) would further increase the sugar levels within the membrane.
Steady-state concentration profiles by forward Euler integration of the reaction-diffusion equations:
with a time-step of 1.5 ms and a lattice spacing of 0.33 µm. Here, the reaction terms describe both the formation of sugars from formaldehyde and their decay as first-order processes. The model did not attempt to capture the complex kinetics of the formose reaction (which quantitatively is poorly understood) but rather are concerned with approximate time scales. It also considered the rate constants ki =1,2 to be space-dependent according to ki(x) = ki Θ(x−w/2), where Θ is the Heaviside function, w the membrane width, D = 10 − 8 cm2/s the constant diffusion coefficient, k1 = 10 − 2 s (half-life 70 s), and k2 varied. These step-like features qualitatively described the absence of sugar production and decay in the cold, neutral portion of the membrane and the presence of these processes within the hot, basic side. Notice that the model disregards the temperature dependence of the diffusion coefficient which in the real system is smaller on the cold side, however, this effect results primarily in a rescaling of w. Simulations were also performed, a few in which the step-like dependence of ki =1,2 was replaced with a smooth sigmoidal dependence generated by an arctangent function. These simulations yielded very similar trends to the model.
Results/Discussion
Formation and Identification of Chemical Gardens
This study produced precipitate tubes at room temperature by steadily injecting aqueous CaCl2 (Fisher) solution into a 100 mM Na2CO3 solution of 1 M sodium silicate. The growth process (Fig. 1A-C) occurs slowly around a rapidly ascending jet and yields a white, cylindrical structure with an average outer radius of ~ 0.4 mm. After production, the fragile tubes were carefully removed from the vessel, washed, and dried.
Production and characterization of hollow silica-CaCO3 chemical gardens. (a-c) Thin-walled tubes (average diameter 0.82 mm, wall thickness 12 µm) grow when CaCl2 solution is injected through a nozzle (thick base) into silicate/carbonate solution. Time between frames 39 s. (d) Scanning electron micrograph of the outer, silica-rich tube wall reveals a microporous texture
Infrared and Raman spectroscopy reveal that the tube wall consisted of silica and calcium carbonate (Figure S1). This is analogous to the calcium carbonate mineral and silica rich environments of alkaline white smoker vents and the lost city hydrothermal fields (Martin et al. 2008). Scanning electron microscopy and energy dispersive spectroscopy showed a smooth, silicon-containing outer surface and a rougher inner surface that is rich in calcium (Figure S2, S3, Table S1). The approximate wall thickness was ~ 12 µm. Micrographs at higher magnification (Figs. 1D, S2) reveal crystallites with diameters of about 1 µm. The powder X-ray diffraction measurements indicated that these microcrystals are primarily calcite, but some aragonite is also detected (Figure S1C).
In the absence of formaldehyde, the vertical tube growth occurred slowly at a steady speed of ~ 0.17 mm/s. Addition of formaldehyde to the reactant solutions did not inhibit the tube growth. To the contrary, the vertical growth velocity v increases with increasing CH2O concentrations in the outer solution. Measurements reveal that the v([CH2O]) dependence is linear with a slope of ~ 0.40 mm/(s M) (Figure S4). Interestingly when grown in the presence of formaldehyde the chemical garden’s crystal composition seems to change, verified by powder x-ray diffractometry (Figure S5). This effect is likely caused by the slow progression of the Cannizzaro reaction (Gardner et al. 1979; Tsujino et al. 1999) in the outer reactant solution. This base-induced process generates formic acid and causes a small but measurable pH decrease of about 0.1.
The Formose Reaction with Silica-CaCO3 Chemical Gardens
Considering the chemical gardens as a model of the precipitates in off-axis alkaline vents, this work further explored whether these tubes catalyzed the formose reaction. For this purpose, calcium chemical garden tubes were transferred from the growth container into an aqueous formaldehyde solution and exposed to elevated temperatures and pressure (maxima 120 °C and 200 kPa) for less than 1 h; additionally, moderate heating time course experiments were performed (maxima 80 °C and 101 kPa). The filtered product solutions were then studied using proton-NMR spectroscopy employing water suppression.
Total mass (m) [a] and relative mole %[b] of monosaccharides as detected by GCMS analysis (Figures S9, S10). The reactant solution had an initial pH of 12, [CH2O] = 1.0 M, and a volume of 3.6 ml. Dry mass of sample analysed was 0.4 mg with 2.4 µg sugars detected yielding 0.6% sugars from formaldehyde. Other signal peaks were detected and likely correspond to branched sugar like species, and organic acids (Figure S9).
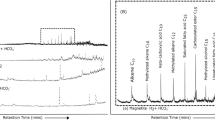
Figure 2 shows representative NMR spectra obtained from such experiments with a starting pH of 12. The basic condition yields a complex product mixture including sugars. Aside from sugars the complex mixture contained Cannizzaro products: formic acid, and methanol.
In order to make sure the sugars were produced by the formose reaction this study used fresh/newly purchased formaldehyde. Additionally, in order to ensure the sugars were produced by the formose reaction, and not due to contaminating sugars, procedural blanks were run under similar conditions with an initial pH of 9 (Figure S6). The NMR measurements for the blanks were carried out using a B600, for proton the detection limits are around 50 nmol and for carbon ~ 1 µmol. The samples contained 500 µl of volume for NMR. The shimming protocols, and ZGGPW5 suppression pulses also increase sensitivity for proton NMR. Long scan methods for carbon NMR were used, these were sensitive enough to detect even heavily de-shielded nuclei (such as formic acid) (Figure S7). This long scan method for carbon was 14 hours long, usually carbon NMR scans would be employed for 3 or 4 hours, which would detect 1–2 micromoles, these were much longer in order to increase the signal to noise ratio. These scans for proton and carbon NMR went into the construction of the HSQC analysis of our procedural blanks (Figure S8). The products observed were Cannizzaro products only and no sugars were identified in the blank solutions (Figure S6, S7, S8).
The presence of multiple sugar signatures and the yellowing of the original solutions (initial pH 12) are clear evidence that the formose reaction occurred in and around the chemical gardens (Figs. 2, S9, S10, S11, Table 1).
In addition, quantitative glycosyl composition analyses which are based on TMS derivatization and highly sensitive GCMS measurements were performed by the Complex Carbohydrate Research Center (Figures S9, S10). This was performed to identify individual sugars that could not be parsed out using NMR alone. The results for the formose reaction reveal the presence of glucose, ribose, xylose, and mannose with the relative abundancies listed in Table 1. GCMS also detected a complex mixture including possible branched sugar like species, organic acids and a C-18 fatty acid (Figures S9, S10). Therefore, the mole % indicates the detectable monosaccharide species after derivatization and do not incorporate the unknowns.
While some formose processes, such as a UV catalyzed process can yield a primary product such as pentaerythritol (Schwartz and De Graaf 1993) this study’s sugar yields lack specificity for a desired sugar species, like ribose, which is typical of most formose reactions (Schwartz and De Graaf 1993). While the required initial concentration of formaldehyde is high, in the absence of needed eutectic freezing or evaporation (Cleaves 2008), hydrothermal vents perhaps obtain concentration from a sedimentary mechanism in or around the vent chimneys (Westall et al. 2018). This fits well into the current paradigm of the formose reaction requiring greater concentration of formaldehyde (Cleaves 2008). The respective low sugar yields are typical for the formose reaction (Reid and Orgel 1967).
The Formose Reaction Catalyzed by Chemical Gardens
Solutions of 1 M formaldehyde pH 12.5 were heated in a 75 °C water bath. The negative controls had no calcium added, the positive control had 0.06 g CaCO3 powder added. The experimental set had 0.06 g of crushed up dried chemical gardens added. Yellowing/reaction times are represented as averages from triplicate runs with time in seconds, error is from the corresponding standard deviations. The chemical garden group showed the quickest reaction times.
Three conditions were used: no calcium, CaCO3 powder added, and chemical gardens added as a heterogenous catalyst, to inspect reaction times for the formose reaction under moderate hydrothermal conditions. At moderate hydrothermal temperatures (80 °C) for 60-minute incubation periods without added chemical gardens or CaCO3 powder, yellowing of the alkaline formaldehyde solutions was not observed and sugar signatures were not detected spectroscopically, the formose reaction did not occur (Table 2). Not only is the reaction made possible by the chemical gardens, but the rate of yellowing increased in the presence of the chemical gardens compared to that of the CaCO3 powder positive controls. The spiking of glycolaldehyde into the positive and negative control formose solutions made them react much faster, similarly to the chemical gardens (Table 2). This is indicating that the first step of the formose reaction, the production of glycolaldehyde is what is being catalyzed by the calcium and silicate membranes of the chemical gardens, as would be expected.
pH Gradients of the Chemical Gardens
The latter experiments establish that at high pH and for hydrothermal conditions, chemical-garden-like structures can help produce sugars from formaldehyde. However, as noted in the introduction, these sugars can be expected to react rapidly (Villamiel et al. 2006). This may be addressed by how the production and the decay of free sugars might unfold in unexpected ways if performed in inorganic membranes. The first difference between a dilute suspension of catalyst particles and a porous membrane is that the latter could constitute a buffer system. To illustrate this effect, the formose reaction was performed with large amounts of calcium carbonate powder and found that for initial pH values between 4 and 10, the final pH equaled 7.7±0.2, which is very close to the pKb of bicarbonate 7.7 establishing a bicarbonate buffer system during the hydrothermal formose process (Figure S12). While this demonstration of buffering works for low flow vents or ponds/lakes, many hydrothermal vents are high flow “smokers”. This stagnant system differs from the conditions in these hydrothermal vent smoker’s walls which experience the fluid motion of the surrounding ocean and the inner vent fluid.
To further explore this situation, calcite membranes were produced in microfluidic devices that allow for a closer inspection of the membrane cross-section (Fig. 3A). These devices have a Y-shaped channel layout and merge streams of CaCl2 (left) and NaOH/Na2CO3/Na2SiO3 solution (right). Due to the low Reynolds number (Re ≈ 1) from solutions flowing in microfluidic spaces and making contact, flows are laminar and form a sharp reactive interface in the middle of the main channel around which solid CaCO3 and silica form. The resulting membrane undergoes unusual growth spurts in the direction of both reactant solutions. Furthermore, the CaCO3 membranes showed large pores with diameters of up to 17 µm. Notice that the pH gradient across the membranes is very large. For the example in Fig. 3, a pH decrease was noted from 11.5 (Na2CO3(aq)) to 3.6 (CaCl2(aq)) over the 880 µm wide membrane. In addition, the inflowing reactant solutions maintain this steep pH gradient over long periods, thus, capturing certain aspects of an active off-axis alkaline vent in an early ocean, which 4.0 Ga ago may have had a pH of 6.6+ 0.6−0.4 (2σ) (Krissansen-Totton et al. 2018).
Inside the precipitate membrane. A Microfluidic device with a CaCO3 membrane formed around the interface between a CaCl2 solution (0.4 M, left stream) and a Na2CO3 solution (0.4 M, right stream). B,C Micrographs of the membrane in the presence of the pH indicators bromothymol blue and phenol red. Field of view: 1.32 mm × 0.35 mm. D Schematics of hydrothermal vent conditions which include steep pH and temperature gradients. Sugar production and sugar decay reaction are expected only within the hot, basic side of the membrane, whereas sugars might accumulate on the cold, near-neutral side
The addition of a pH indicator to both reactant solutions allows for probing of the transmembrane pH profile. Figure 3B, C illustrates this approach for the examples of bromothymol blue (yellow/blue, pH 7.6) and phenol red (yellow/purple, pH 8.2), respectively. It is observed that the color changes occur over distances much smaller than the membrane width and at positions that correspond to 11% and 18% of the total width, determined by time lapse microscopy. It is concluded that the pH profile, while steep, varies steadily across the membrane without forming a pronounced shoulder or plateau. In the case of hydrothermal vents, this externally driven gradient is complemented by a steep temperature gradient of up to 1 K/µm. This situation is further complicated by the heat and mass transport around and within the vent structure that in the isothermal, microfluidic system is a simple laminar flow along the membrane. Furthermore, one can expect that the removal of hydroxide ions and heat, through ocean currents is more effective than in the experimental/conceptual model.
Modeling/Numerical Simulations to Address Sugar Degradation/Accumulation
The complexity of the membrane material in hydrothermal vents and the uncertainties concerning the temperature and chemistry of the Hadean ocean set limitations for rigorous modeling efforts. Nonetheless, it is suggested that simple models can provide useful insights into the involved reaction-transport processes and specifically into the question of sugar production and decay (Fig. 3D). For this purpose, the membrane (width w) was modeled as two adjacent, equally wide layers. The outside layer is cold and has near neutral pH, whereas the interior layer is hot and basic. Sugar production and decay occur only in the second layer according to the irreversible, first-order reactions F → S → W where the three chemical species represent formaldehyde, sugar, and waste products, respectively. These species diffuse across both layers with a constant diffusion coefficient D. The important boundary conditions for the simulations are conditions that enforce constant concentrations at x = 0 (the ocean) and x = w (the interior vent fluid). These concentrations are zero except for formaldehyde that enters the membrane at x = 0 without diminishing its concentration in the ocean. It is emphasized that these boundary conditions cause the maximal loss of sugars into the surrounding solutions and do not consider a possible accumulation of sugars in the vent fluid or ocean water.
Figure 4A shows four representative steady-state calculations for this model. The blue curve describes the normalized formaldehyde concentration across the membrane, which is independent of the decay rate constant k2. The red curves are normalized sugar concentration profiles for four different k2 values. These profiles have a triangular character and peak near the transition line between the cold/neutral and hot/basic regions (dashed line). Notice that for low decay rates the two regions hold nearly the same total amount of sugar, however, as the rate constant k2 is increased, the system transitions to an asymmetric sugar profile and now sugars accumulate primarily in the cold/neutral zone. Figure 4B shows that this transition coincides with a decrease of the maximal sugar concentration which equals approximately 5–10% of the formaldehyde concentration in the ocean. For ratios k1 / k2 between about 0.1 and 10, this decreased yield depends only weakly on the membrane width, which varied between 25 and 150 µm. The transition to an asymmetric accumulation-production mode, however, depends on the membrane width and occurs already at large k1 / k2 (slow decay) in thick membranes (inset of Fig. 4B).
Numerical simulations of the formose reaction and the sugar decay in thin membranes. Formaldehyde enters the system from the left and reactions occur only in the right half. a Normalized estimated parameters, steady-state concentration profiles of formaldehyde (blue) and sugar (red), the latter for sugar decay rate constant is assumed to be k2 = 0.001, 0.01, 0.1, 1.0 s1 varied by magnitude. b Maximal steady-state sugar concentrations in percent of the inflowing formaldehyde concentration computed for four different membrane thicknesses. The inset shows the percentage of sugars in the left half of the system as a function of log(k1/k2) and for the same four membrane widths. The sugar production rate constant k1 = 10− 2 s− 1 is kept constant
A Note for Researchers Studying a Warm Pond/Dry Land Hypothesis
For a low flow or stagnant system like a warm pond or mild geyser on dry land stable buffers may form in the presence of carbonate minerals (Figure S12) and when exposed to a CO2 dominant atmosphere (Toner and Catling 2020). Coincidentally, these high CO2 environments could provide protective carbonate/bicarbonate buffers that may also provide a source of formaldehyde for the formose reaction. Evaporation of these ponds could concentrate the formaldehyde as well. It is with all this in mind that the formose reaction is a plausible prebiotic chemistry given that there may be multiple environments that support the production and accumulation, or protection, of formose sugars.
Conclusions
It has been shown that silica-CaCO3 based chemical gardens catalyze the formose reaction (Tables 1 and 2). This is a good example of a vent material analogue acting as a heterogenous catalyst for a prebiotically relevant reaction. This also provides an experimental model for systematic studies of prebiotic sugar production and accumulation in membrane systems that are exposed to chemical and thermal gradients, and disequilibrium, such as the chemical garden’s structure and properties.
Earlier criticism that the formose reaction has no prebiotic relevance due to the short lifetime of sugars under hot and/or basic conditions should perhaps be qualified because precipitate membranes are subject to an externally maintained disequilibrium. This state could allow the dynamic accumulation of free sugars and even the spontaneous partitioning of the membrane into sugar producing and sugar accumulating regions.
These conditions indicate that environments such as alkaline submarine hydrothermal vents are a plausible location for the formose reaction. I believe this to be in support of an RNA world, which is a sturdy and well buttressed hypothesis standing the test of time (Neveu et al. 2013).
Change history
15 September 2020
Editor's Note: Authorship of this manuscript is currently under dispute. Appropriate editorial action will be taken once investigation into this matter is complete and all parties have been given an opportunity to respond in full.
28 November 2023
Editor’s Note: Following an investigation, it was found that the issue of authorship has been resolved.
References
Ajandouz EH, Desseaux V, Tazi S, Puigserver A (2008) Effects of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem 107:1244–1252
Branscomb E, Biancalani T, Goldenfeld N, Russell M (2017) Escapement mechanisms and the conversion of disequilibria, the engines of creation. Phys Rep 677:1–60
Breslow R (1959) On the mechanism of the formose reaction. Tetrahedron Lett 21:22–26
Butlerow A (1861) Bildung einer zuckerartigen Substanz durch Synthese. Liebigs Ann Chem 120:295–298
Cleaves II, HJ (2008) The prebiotic geochemistry of formaldehyde. Precambr Res 164(3–4):111–118
Cody GD, Heying E, Alexander CMO, Nittler LR, Kilcoyne ALD, Sandford SAR, Stroud M (2011) Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. USA 108, 19171–19176
Gardner Swain C, Powell AL, Sheppard WA, Morgan CR (1979) Mechanism of the Cannizzaro reaction. J Am Chem Soc 101:3576–3583
Horecker BL (1976) The biochemistry of sugars. Int Z Vitamin-und Ernahrungsforschung 15:1–21
Hughes EAB, Williams RL, Cox SC, Grover LM (2017) Biologically analogous calcium phosphate tubes from a chemical garden. Langmuir 33:2059–2067
Krissansen-Totton J, Arney GN, Catling DC (2018) Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc Natl Acad Sci U S A 115(16):4105–4110
Larralde R, Robertson MP, Miller SL (1995) Rates of decomposition of ribose and other sugars: implications for chemical evolution. Proc Natl Acad Sci 92:8158–8160
Löb WZ (1906) Elektrochem Angew Phys Chem 12:282
Martin W, Russell MJ (2007) On the origin of biochemistry at an alkaline hydrothermal vent. Phil Trans Roy Soc B 362:1887–1925
Martin W, Baross J, Kelley D, Russell MJ (2008) Hydrothermal vents and the origin of life. Nat Rev Microbiol 6:805–814
Möller FM, Kriegel F, Kieß M, Sojo V, Braun D (2017) Steep pH gradients and directed colloid transport in a microfluidic alkaline hydrothermal pore. Angew Chem Int Ed 56:2340–2344
Neveu M, Kim HJ, Benner SA (2013) The “strong” RNA world hypothesis: Fifty years old. Astrobiology 13(4):391–403
Orgel LE (2004) Water soluble nanoparticles from PEG-based cationic hyperbranched polymer and RNA that protect RNA from enzymatic degradation. Crit Rev Biochem Mol Biol 39:99–123
Pinto JP, Gladstone GR, Yung YL (1980) Photochemical production of formaldehyde in Earth's primitive atmosphere. Science 210:183–185
Reid C, Orgel LE (1967) Model for origin of monosaccharides: synthesis of sugars in potentially prebiotic conditions. Nature 216:455
Ricardo A, Carrigan MA, Olcott AN, Benner SA (2004) Borate minerals stabilize ribose. Science 303:196
Ruiz-Mirazo K, Briones C, de la Escosura A (2014) Prebiotic systems chemistry: new perspectives for the origins of life. Chem Rev 114:285–366
Russell MJ, Barge LM, Bhartia R, Bocanegra D, Bracher PJ, Branscomb E, Kidd R, McGlynn S, Meier DH, Nitschke W, Shibuya T, Vance S, White L, Kanik I (2014) The drive to life on wet and icy worlds. Astrobiol 14:308–343
Schwartz AW, De Graaf RM (1993) The prebiotic synthesis of carbohydrates: a reassessment. J of mol Evol 36:101–106
Toner JD, Catling DC (2020) A carbonate-rich lake solution to the phosphate problem of the origin of life. Proc Natl Acad Sci 117(2):883–888
Tsujino Y, Wakai C, Watubayashi N, Nakahara M (1999) Noncatalytic Cannizzaro-type reaction of formaldehyde in hot water. Chem Lett 4:287–288
Villamiel M, del Castillo MD, Corzo N, Hui YH (2006) Food biochemistry and food processing, Wiley-Blackwell, Hoboken
Weiss AH, Lapierre RB, Shapira J (1970) Homogeneously catalyzed formaldehyde condensation to carbohydrates. J Catal 16:332–347
Wen JS, Pinto JP, Yung YL (1989) Ions of martian origin and plasma sheet in the martian magnetosphere: initial results of the TAUS experiment. J Geophys Res 94:14957–14970
Westall F, Hickman-Lewis K, Hinman N, Gautret P, Campbell KA, Bréhéret JG, … Kee TPA (2018) Hydrothermal-sedimentary context for the origin of life. Astrobio 18(3):259–293
Acknowledgments
I thank Parastoo Azadi at the Complex Carbohydrate Research Center for her assistance with sugar analyses and writing. I thank Qingpu Wang for his assistance with microfluidic experiments and writing. I thank Oliver Steinbock for his assistance with simulations/modeling, support, and writing. Special Thanks to Chengi Zhang for her assistance with NMR.
Funding
This work was supported by the US National Science Foundation under grant DMR-1609495. Work at the Complex Carbohydrate Research Center was funded by US Department of Energy grant DE-SC0015662.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 1530 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omran, A. Plausibility of the Formose Reaction in Alkaline Hydrothermal Vent Environments. Orig Life Evol Biosph 53, 113–125 (2023). https://doi.org/10.1007/s11084-020-09599-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-020-09599-5