Abstract
Hepatocellular carcinoma (HCC) is a leading cancer whose incidence is increasing globally. In spite of advances in medical and surgical therapies, its prognosis remains deprived. Developing methods for early diagnosis is essential for better treatment outcomes. In this pilot study, laser-induced linear polarization was adopted for the characterization of total RNA samples obtained from hepatocellular carcinoma (HCC) patients against healthy volunteers. Total RNA samples were illuminated with non-ionizing laser source of 632.8 nm; the scattered light signatures were captured and processed using hyperspectral camera. Scattered light signature was found to depend on polarizabiltiy, size, shape, and chemical structure of scattering macromolecules. DNA (control) demonstrated the highest scattered intensity; with intense fringes compared with RNA (control). Meanwhile RNA (HCC) demonstrated the lowest scattered intensity as well as the lowest fringe spacing compared with RNA (control). For instance, at 380 fringe spacing DNA (Control), RNA (Control), RNA (HCC) demonstrated scatted light intensity of 0.049, 0.039, and 0.034, respectively. Novel column mean calculation was conducted for hyperspectral images at 632.8 nm to assess scattered signal intensity and fringe spacing. The scattered RNA signal could secure characteristic finger-print fringes; that are candidate for facile HCC early diagnosis. To the best of our knowledge, this is the first time to report an optical methodology based on linear polarization for HCC facile diagnosis as a pilot study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
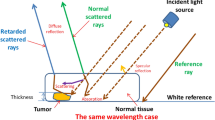
Hepatocellular carcinoma (HCC) develops when cells divide, replicate, and spread uncontrollably at high rate which may be due to epigenetic alterations and mutations affecting the cellular machinery (Hartke et al. 2017; Gilles, Garbutt et al. 2022). Primary liver cancer as HCC is most frequently found in those with cirrhosis or chronic liver disease and is most closely associated with exposure to poisons such as alcohol, aflatoxin, or pyrrolizidine alkaloids as well as persistent viral hepatitis infection. HCC risk factors include metabolic syndrome and non-alcoholic steatohepatitis (NASH) (Forner, Reig et al. 2018; Ronot, Purcell et al. 2019). Nevertheless, up to 25% of HCC patients have no history of chronic liver disease. HCC prognosis is poor because only 10–20% of HCC can be removed completely using surgery; this may be due to late presentation and diagnosis (Chen, Xu et al. 2020; Ren, Ma et al. 2020). Sophisticated spectroscopic techniques can be adopted for HCC diagnosis (Sheil, Khan et al. 2021). However, these methods lack simplicity and dependability (Sheil, Khan et al. 2021). Improvement in medical imaging could help in developing new methods of early HCC diagnosis (Zhang, Wang et al. 2022). HCC can be diagnosed by ultrasound, computerized tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI). Developing methods for early cancer diagnosis gives the best chance for successful treatment (Clark, Maximin et al. 2015; Jiří, Igor et al. 2020). Light scattering can secure facile approach for early cancer diagnosis. Careful analysis of the scattered light can yield detailed information about the scattering material (Shen, Zhang et al. 2021). When an oscillating electric field is applied on macromolecules; it provokes dipole moment via positive and negative charge partial separation. The developed dipole moment can oscillate at resonant frequency with the incident electric field (Chorfi, Ayadi et al. 2020). The developed dipole moment can switch energy with incident electric field (Li, Zou et al. 2019). It can re-emit electric field at the same frequency known as Raleigh scattering (Zhan, Fang et al. 2022). If the scattering centers are connected into one larger particle; then the scattered light would add coherently (Wang, Zhang et al. 2019). If the two scattering centers are separated, the scattered light adds incoherently (Fig. 1).
The size, polarizability, chemical composition, and concentration of macromolecules all influence scattered light intensity in a linear fashion (Ligiero, Fernandes et al. 2022; Tuniyazi, Mu et al. 2023). Therefore linear spectroscopy can offer reliable information about macromolecule chemical structure for instance DNA and RNA disorder (El-Sharkawy, Elbasuney et al. 2021).
In this pilot study, RNA disorder due to HCC was investigated using laser-induced light scattering. Total RNA samples obtained from HCC patients were investigated against healthy volunteers. Total RNA samples were illuminated with non-ionizing laser source of 632.8 nm. The scattered light signature was captured and processed using hyperspectral camera. RNA (HCC) experienced characteristic scattered can be adopted for early HCC diagnosis.
2 Subjects and methods
2.1 Subjects
Before receiving any chemotherapy or radiation, de novo diagnosed male HCC patients (n = 10) from the clinical oncology clinic at Ain Shams University Hospitals in Cairo, Egypt, participated in the study. The European Association for the Study of the Liver’s (EASL) evaluation standards and histologic sample served as the foundation for the diagnosis. Ten healthy male volunteers of similar age served as the control group. Each patient gave their informed agreement, and the study was authorized by the Ain Shams University Hospitals’ Ethical Committee for Research. Additionally, the study was conducted in accordance with the guidelines and suggestions of the Helsinki Declaration.
2.2 Laboratory analysis
To prepare whole blood for total RNA purification and complete blood count (CBC) testing, blood samples were drawn into two EDTA vacutainers.
2.3 Complete blood count (CBC) analysis
For the measurement of hemoglobin (Hgb), red blood cell (RBC) count, total leukocyte count (TLC), and platelet count (PLT), the Z2TM Coulter Counter®, Analyzer, Coulter Electronics, USA was utilized.
2.4 Genomic DNA extraction and total RNA purification
Following the manufacturer’s instructions, genomic DNA was extracted using the QIAamp DNA Mini Kit technique from Qiagen in Hilden, Germany. DNA quantification and purity were then determined using the NanoDrop® (ND)-1000 spectrophotometer from NanoDrop Technologies, Inc. in Wilmington, Delaware, USA. Using a QIAamp RNA blood micro kit (Qiagen, Hilden, Germany), total RNA was extracted and purified in accordance with the manufacturer’s instructions.
2.5 Linear signal measurement
The proposed optical light scattering system consists of four main elements: laser source working at 632.8 nm with 10 mw power, sample holder, hyperspectral (HS) camera, and the signal/image processing and analysis software. The investigated sample was stimulated via 10 mw laser source working at 632.8 nm. The scattered light was collected via optical length with focal length 10 cm to high speed, highly sensitive hyperspectral camera HS camera (Resonon, Pika XC2, USA) with 600-line scans, 1.3 nm resolution, 12 bits of Bit depth, maximum frame rate 165 fps, spatial channels of 1600, and spectral channels equal to 462. The employed camera was integrated with a lens (Schneider,6 mm, Focal point = 2.1, Range 400∼1000 nm) (Fig. 2).
The reconstructed cube image from the camera was processed and presented using digital image processing algorithm utilized DADiSP 6.5 international software.
2.6 Data normalization
To avoid the spectral non-uniformity of the illumination device and the influence of the dark current, the radiance data were normalized to present the radiance of the specimen. To achieve this pre-processing step, the radiance of a standard white reference board was placed in the field of view. The dark current was measured by keeping the camera shutter closed. The raw data was corrected using Eq. (1).
Where I raw (λ) is the raw data radiance value for a specific pixel, I dark (λ) and I white (λ) are the radiance values of the dark current and the white reference board for each spectral band, respectively, and R (λ) is the computed reflectance value for each wavelength.
3 Result and discussion
3.1 Demographic information and participant clinical characteristics
Table 1 provides a summary of the participant’s clinical and demographic information.
3.2 Hyperspectral images of linear polarization signals
Light scattering arises from the presence of heterogeneities within a bulk medium due to physical inclusion, and fluctuations in dielectric constant from random thermal motion. The difference in the structure, molecules size, shape, and concentration of the DNA and RNA could withstand the change in the scattered light intensity as well as the formation of fringe diffraction pattern. Moving average filter processing was used to remove noise from the hyperspectral pictures that were taken at 632.8 nm. The electrostatic force of DNA’s molecules and its double helix shape caused DNA (Control) to scatter far more intensely than RNA (Ma, Shi et al. 2023). The difference in the scattered light intensity and position could lead to change in fringe spacing and diffraction formation (Fig. 3). X and Y axes represent the relative position of the scattered signal at 632.8 nm; they are dimensionless.
The scattering pattern of RNA (Control) was uniform around the main laser beam in both sides; this could be ascribed to the stability of the molecules structure. On the other hand, RNA (HCC) experienced change in the contrast, as well as the distribution around the main beam. The change in light scattering pattern and fringes could offer novel finger-print for HCC diagnosis. While DNA can secure coherence interference of scattered light; RNA can expose incoherent interference. The difference in scattered light interaction (coherent/incoherent) can result in different scatted light intensity and fringes. Furthermore, scattered light signal could change with macromolecule size, chemical structure, and polarizability. These features can secure concrete platform for facile HCC diagnosis.
3.3 Fringe spacing of linear polarization signals
The contour of hyperspectral images at 632.8 nm was performed to identify the intensity, and light scattering position and to evaluate the fringe spacing. Whereas DNA (Control) and RNA (Control) demonstrated diffraction formation of symmetric pattern; RNA (HCC) demonstrated low scattered light intensity and unsymmetrical distribution around the centre (Fig. 4).
The column means of the hyper-spectral image for DNA (Control), RNA (Control), RNA (HCC) was calculated to estimate fringe spacing modality. Whereas DNA (Control) demonstrated the highest scattered intensity; with intense fringes compared with RNA (Control). On the other hand, RNA (HCC) demonstrated the low scattered intensity as well as the low fringe spacing compared with RNA (Control) (Fig. 5).
It is apparent that there are a difference between scattered light intensity over the band 360: 400 fringes spacing. For instance, at 380 fringes spacing:
-
DNA (Control) demonstrated scatted light intensity of 0.049.
-
RNA (Control) demonstrated scattered light intensity of 0.039.
-
RNA (HCC) demonstrated scattered light intensity of 0.034.
It is generally acknowledged that as particle size increases, scattered light intensity also rises, exhibiting faster oscillations (fringes). It may be possible to connect changes in molecular size and shape (Fig. 1), chemical structure, and material polarizability with variations in scattered light intensity and fringes. The nucleotides represented in two long strands that form a double helix DNA and single strand RNA were mixture of molecules (scattering particles); scattering oscillation signal was spectra superposition as represented in Fig. 5. The variation of scattered light intensity and fringes with variation in molecular size was demonstrated in Fig. 6.
Correlation of particle size with scattering oscillation (Fringes) revealed that as particle size increase more rapid oscillation would occur. Scattered signal spacing could be adopted to estimate the size of scattering particles; modulation depth can provide information about the number of such scattering particles.
4 Conclusion
HCC development is accompanied with RNA disorder. Scattered light of RNA (HCC) can secure promising feature for HCC diagnosis. Scattered light signature was found to depend on macromolecule polarizability, size, shape, and chemical structure. Whereas DNA (Control) demonstrated the highest scattered intensity; with intense fringes compared with RNA (Control). RNA (HCC) demonstrated the low scattered intensity as well as low fringe spacing compared with RNA (Control). The scattered RNA signal could secure characteristic finger-print fringes candidate for facile HCC early diagnosis as a pilot study. Fluorescence and Raman spectroscopy depend on the macromolecule chemical structure which is very similar in all investigated samples. Subsequently it could not provide substantial signal differentiation for early cancer diagnosis.
Data availability
The corresponding author is willing to provide the datasets used and analysed during the current work upon reasonable request.
References
Chen, V.L., Xu, D., et al.: Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, determination of prognosis, and Disease Monitoring: A systematic review. Clin. Gastroenterol. Hepatol. 18(13), 2879–2902e2879 (2020)
Chorfi, H., Ayadi, K., et al.: Contribution Study cancer Tissues Light Scattering Optik. 221, 165094 (2020)
Clark, T., Maximin, S., et al.: Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging diagnosis, Response Assessment, and treatment. Curr. Probl. Diagn. Radiol. 44(6), 479–486 (2015)
El-Sharkawy, Y.H., Elbasuney, S., et al.: Total RNA nonlinear polarization: Towards facile early diagnosis of breast cancer. RSC Adv. 11(53), 33319–33325 (2021)
Forner, A., Reig, M., et al.: Hepatocellular Carcinoma Lancet. 391(10127), 1301–1314 (2018)
Gilles, H., Garbutt, T., et al.: Hepatocellular Carcinoma Crit. Care Nurs. Clin. North. Am. 34(3), 289–301 (2022)
Hartke, J., Johnson, M., et al.: The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn. Pathol. 34(2), 153–159 (2017)
Jiří, T., Igor, K., et al.: Hepatocellular carcinoma future treatment options. Klin. Onkol. 33(Supplementum 3), 26–29 (2020)
Li, T., Zou, L., et al.: Non-invasive diagnosis of bladder cancer by detecting telomerase activity in human urine using hybridization chain reaction and dynamic light scattering. Anal. Chim. Acta. 1065, 90–97 (2019)
Ligiero, C.B.P., Fernandes, T.S., et al.: Influence of particle size on the SARS-CoV-2 spike protein detection using IgG-capped gold nanoparticles and dynamic light scattering. Mater. Today Chem. 25, 100924 (2022)
Ma, B., Shi, Y., et al.: A novel method for the direct detection of light stabilizer tinuvin 622 in polymer additives by gel permeation chromatography combined with multi-angle laser light scattering. Talanta. 253, 123896 (2023)
Ren, Z., Ma, X., et al.: Diagnosis, Therapy, and Prognosis for Hepatocellular Carcinoma. Anal Cell Pathol (Amst) 2020: 8157406. (2020)
Ronot, M., Purcell, Y., et al.: Hepatocellular Carcinoma: Current imaging modalities for diagnosis and prognosis. Dig. Dis. Sci. 64(4), 934–950 (2019)
Sheil, C.J., Khan, U., et al.: Two-photon polymerization nanofabrication of ultracompact light scattering spectroscopic probe for detection of pre-cancer in pancreatic cyst. Opt. Lasers Eng. 142, 106616 (2021)
Shen, R., Zhang, J., et al.: Dynamic light scattering and fluorescence dual-signal sensing of cancer antigen-125 via recognition of the polymerase chain reaction product with gold nanoparticle probe. Anal. Chim. Acta. 1145, 87–94 (2021)
Tuniyazi, A., Mu, T., et al.: Bayesian neural networks enabled snapshot polarized light scattering spectroscopy with uncertainty quantification. Opt. Lasers Eng. 160, 107298 (2023)
Wang, J., Zhang, J., et al.: Strand displacement amplification-coupled dynamic light scattering method to detect urinary telomerase for non-invasive detection of bladder cancer. Biosens. Bioelectron. 131, 143–148 (2019)
Wyatt, P.J.: Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta. 272(1), 1–40 (1993)
Wyatt, P.J.: Differential light scattering and the measurement of molecules and nanoparticles: A review. Anal. Chim. Acta: X: 100070. (2021)
Zhan, S., Fang, H., et al.: M13 bacteriophage as biometric component for orderly assembly of dynamic light scattering immunosensor. Biosens. Bioelectron. 217, 114693 (2022)
Zhang, X., Wang, Z., et al.: NIR light-powered halloysite-based nanomotors for CT imaging diagnosis and synergistic chemo-photothermal cancer therapy. J. Ind. Eng. Chem. 116, 180–190 (2022)
Funding
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
YHE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. SE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. SMR suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. MAS suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. GSE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing.
Corresponding authors
Ethics declarations
Ethics approval
According to the Helsinki Declaration, all experimental and investigative trials were authorized by Cairo University’s Ethical Committee of Research (P.T.REC/009/003144). Before data collection began, all participants read and signed two copies of a permission form.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors affirm that they have no financial or other conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sharkawy, Y.H., Elbasuney, S., Radwan, S.M. et al. Novel laser-induced linear polarization for facile diagnosis of hepatocellular carcinoma. Opt Quant Electron 56, 709 (2024). https://doi.org/10.1007/s11082-024-06486-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-024-06486-5