Abstract
Glasses from two varieties of (LiF-B2O3) and (LiF-P2O5) systems with equal 50 mol % for each partner together with samples doped with 1 or 2% Bi2O3 were synthesized by melting–annealing mechanism. Multiple optical, FTIR and photoluminescence spectral measurements were carried out. Optical spectra of Bi2O3—doped glasses exhibit UV-near visible peaks owing to the specific absorption of Bi3+ ions. FTIR spectra elucidate the presence of characteristic vibrational bands from (LiF-B2O3) glass due to (BO3) and (BO3F) groups beside (LiF4) groups while the fluorophosphate glasses (LiF-P2O5) show vibrations due to PO4 and (PO3F) groups and also the possible sharing of (LiF4) groups. Photoluminescence spectral data reveal extended emission from about 375 to 700 nm and is more prominent in (LiF-B2O3) system than (LiF-P2O5) system. The thermal expansion data reveal similar behavior and the derived transition and softening temperatures are slightly lower in (LiF-P2O5) glasses than (LiF-B2O3) glasses.
Similar content being viewed by others
1 Introduction
The introduction of alkali metal fluorides into any conventional silicate, phosphate, or borate glass results in a blend of different glasses with the good optical properties of fluoride glasses and the higher mechanical and thermal stability of partner oxide glasses (Lucas 1989; Möncke and Ehrt 2019). The term fluoride glass refers to particulate vitreous materials belonging to the general group of halide glasses in which the anions are derived from elements of Group VII of the Periodic Table of the Elements, namely fluorine, chlorine, bromine and iodine. Glass materials based entirely on inorganic halides are known to be purely synthetic and their development only started in the mid-1970s (Möncke and Ehrt 2019). Examples of these fluorides are BeF2 and ZrF4 which are known to possess a larger transmission range and are of interest in laser windows, infrared domes, lenses, filters, and laser hosts (Möncke and Ehrt 2019). Fluoride phosphate glasses (or fluorophosphate glasses) have been studied for their short- and mid-range structures for over 50 years and have been summarized by several glass scientists e.g. (Möncke and Ehrt 2019; Kondakis et al. 2010; Ehrt 2015).
Similarly, the interest and progress in fluoroborate glasses have increased since the pioneer and successive studies of Shelby et al. e.g. (Gressler and Shelby 1988; Shelby and Ortolanor 1990; Shelby and Baker 1998) on the network building structure and characteristics of these glasses. In such mixed fluorophosphate and fluoroborate glasses, the most accepted additional formed groups; namely (PO3F) or (BO3F) are expected to be formed as tetrahedral structural groups.
The introduction of Bi2O3 to glasses generally improves their optical properties including the increase of their refractive indices and causes distinct stability against high doses of gamma irradiation due to the heavy atomic mass of Bi2O3 which decreases the mobility of free d-electrons during the irradiation process (Dumbaugh and Lapp 1992; Donald 1993; Bishay and Maghrabi 1969; Stehla et al. 1998; ElBatal and Nucl. 2007; ElBatal et al. 2007). Bi2O3 can form binary glasses with B2O3 or SiO2 with a high percent reaching 85 wt %. This behavior is very similar to that of PbO and many authors have reached the conclusion that bismuth ions can be existed in glasses both as network modifiers and as network former especially at a high percent of Bi2O3 by forming constitutional network BiO3 units beside BiO6 groups (Bishay and Maghrabi 1969; Stehla et al. 1998; ElBatal 2007; ElBatal et al. 2007, 2020; Ouis et al. 2020).
The main objective of this study is to prepare varying Bi2O3-doped (1, 2 wt%) within the two host glasses of (LiF 50%–B2O3 50%) and (LiF 50%–P2O5 50) and to find out the behavior of bismuth ions in these two different fluoride glasses through measuring their collective optical, FTIR and photoluminescence spectra. A further study is undertaken to measure the thermal expansion properties of all the prepared undoped and Bi2O3—doped glasses from the two mentioned systems (LiF-B2O3) and (LiF–P2O5). The collected data would be expected to introduce new data of these two specific fluoroborate and fluorophosphate glass with unfamiliar high LiF content (50 mol%).
2 Experimental details
2.1 Preparation of the glasses
The glasses were synthesized from laboratory chemical reagents. The two hosts studied glass systems include (LiF B2O3) and (LiF-P2O5) with 50 mol% from each partner. The laboratory chemicals used in the preparation of the studied glasses include: orthoboric acid (H3BO3) for B2O3 and ammonium dihydrogen orthophosphate (NH4H2PO4) for P2O5 beside both LiF and the dopant oxide (Bi2O3) was introduced as such. The melting of the weighed batches was carried out into covered platinum crucibles for the fluoroborate glasses and covered porcelain crucibles for the fluorophosphate to minimize the volatilization of fluoride. The batches were melted at 1000–1050 °C for 60 min under atmospheric conditions in a SiC-heated furnace (Vecstar, UK).
The crucibles were rotated at intervals to reach complete melting and homogeneity. The finished melts were poured into warmed stainless steel molds with the required dimensions. The prepared glasses samples were transferred to an annealing muffle regulated at 285 °C for the fluorophosphate and 320 °C for fluoroborate. The annealing muffle was left to cool after 1 h to room temperature with the glassy samples inside.
2.2 Optical absorption measurements
The UV–visible spectra were measured for polished samples of equal thickness (2 mm ± 0.1 mm) using a recording spectrophotometer (type Jasco, Japan)within the range 200–1100 nm.
2.3 FT infrared spectral investigations
The FTIR spectra of the prepared glasses were measured as powders at room temperature within the wavenumber 400–4000 cm−1 using a recording FTIR spectrometer (type Bruker, Vertex 8 V, Germany). The measurements were done with a resolution of 4 cm−1.
2.4 Photoluminescence spectral measurements
PL spectral measurements were recorded at room temperature under different excitation wavelengths in the spectral range 500–700 nm using a fluorescence spectrophotometer (type Jasco-FP-6500, Japan) equipped with a xenon flash lamp as the excitation light source. The scan speed is 0.1 s step-1 with a step wavelength of 0.25 nm and a slight width of 0.2 nm.
3 Results and discussions
3.1 FTIR spectral measurements of the prepared glasses
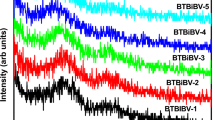
Figure 1 illustrates the FTIR of the undoped glass as well as the two Bi2O3 doped LiF-B2O3 glasses. Infrared spectroscopic results show nearly repeating spectral infrared curves, revealing the same characteristic vibrational bands.
The base undoped LiF-B2O3 glass reveals an FTIR spectrum composed of the following spectral items:
-
(a)
A small far-IR peak located at 486 cm−1 is identified.
-
(b)
A second medium band is observed with two connected peaks at 583 and 613 cm−1.
-
(c)
A broad and highly intense band is identified extending from 800 to 1180 cm−1 with several attached peaks at about 920 and 1047 cm−1.
-
(d)
The second broad and distinct band is observed to be extending from about 1200 to 1550 cm−1 with a distinct peak at 1477 cm−1 followed by a small curvature at about 1650 cm−1.
-
(e)
The rest of the IR spectrum reveals small peaks at 2000, 2500, 2850, and 2930 cm−1 and succeeded by a broad medium near IR band showing two peaks at 3440 and 3569 cm−1.
Figure 2 illustrates the FTIR spectra of the glasses from the system (LiF-P2O5) consisting of the undoped and the two Bi2O3—doped glasses. All the IR curves reveal vibrational condensed peaks within the region 400—1350 cm−1 and followed by separate peaks at about 1640, 2450, and ended by a broad near IR band centered at about 2440 cm−1.
The identified vibrational peaks from the two studied systems due to the unfamiliar composition of the glasses containing high LiF (= 50 mol%) are condensed nature due to possible of mixed groups. It is expected that extra or additional groups beside (BO3) groups and tetrahedral (BO3F) and (PO3F) groups besides the formation of some Bi–O units. It has been profitable to conduct a deconvolution process to the IR base glass in each system together with the IR of the highest Bi2O3 glass. The deconvoluted spectra will be expected to identify hidden or overlapped IR absorption peaks.
Figures 3, 4, 5 and 6 reveal the deconvoluted IR spectra of the selected glasses from the two systems (LiF-B2O3) and (LiF-P2O5). Inspection of Figs. 3 and 4 indicates that the common deconvoluted peaks in the two IR curves of undoped and high Bi2O3 -doped lithium borate glasses are: 405, 486, 494, 563, 598, 613, 711, 902, 912, (1052–1057), 1119, 1188, 1398, 1411, 1488, (1637–1643).
From Figs. 5 and 6 the derived common deconvoluted peaks in the IR curves of undoped and high Bi2O3 -doped lithium fluorophosphate glasses (LiF-P2O5) are: 407, 425, 457,532–541,605–606,712–722, 860, 921–924,965,1026–1050, 1088, 1130–1144, 1387, 1423, 1478, 1531, 1637 cm−1.
The specified extended FTIR spectrum of the undoped LiF-B2O3 glass is explained on the following basis (Shelby and Baker 1998; The structure of vitreous and liquid boron oxide 1962; Kamitsos et al. 1990; Kamitsos 2003; Doweidar et al. 2012).
-
(a)
It is established that the identified vibrational bands and peaks deduced from IR measurements of glasses refer to network structures of such glasses which are originating from the stretching and bending vibrations of the structural building groups which are dependent or related to the detailed chemical composition and their arrangements or housing within the networks.
-
(b)
The first glassy system studied is based on the composition of lithium fluoride borate or lithium fluorophosphate (LiF 50-B2O3 50 mol %) and the expected formed groups include tetrahedral, fluoro-borate (BO3F) and triangular borate (BO3) groups as evidenced in many publications (Möncke and Ehrt 2019; Shelby and Baker 1998; Ouis et al. 2020; Doweidar et al. 2012).
The resultant IR spectra reveal and confirm these previous postulations. Also, they agree with the assumption of the ability of LiF to act in a similar way as Li2O in the progressive transformation of some BO3 to BO3F until a certain percent. Owing to the existence of LiF in measurable equal percent to B2O3 (50 mol %) in the host glass, it is to be assumed that the possibility arises from extra LiF to form additional (LiF4) groups. This postulation is in agreement with the previously published work by Doweidar et al (Doweidar et al. 2012) on CaF2–B2O3 glasses. These authors have assumed that at a high percent of CaF2 (40-45 mol%) the formation of tetrahedral (CaF4) groups is expected as the same to be identified in crystalline CaF2 and this behavior stabilizes the glasses containing high fluoride content.
-
(c)
Regarding the identified IR spectra, it is recognized that the vibrational bands and peaks can be assigned as follows(The structure of vitreous and liquid boron oxide 1962; Kamitsos et al. 1990; Kamitsos 2003; Doweidar et al. 2012):
-
(i)
The multi-small far IR peaks at 406–500 cm−1 can be correlated to vibrations of monovalent (Li.4+) cations in their characteristic sites as derived by many glass scientists (The structure of vitreous and liquid boron oxide 1962; Kamitsos et al. 1990; Kamitsos 2003; Doweidar et al. 2012; Sigel and Ginther 1968; Sigel and in 1977; Duffy 1997; ElBatal and Ouis 2011)
-
(ii)
The identified vibrational peaks within the range 600–750 cm−1 are correlated with bending vibrations of different borate groups
-
(iii)
The observed strong highly intense vibrational peaks within the wavenumber 800–1200 cm−1 are attributed to originate from vibrational of tetrahedral fluoroborate (BO3F) beside the possibility of the sharing of some (LiF4) groups leading to the higher intensities of such middle absorption region at (800–1200 cm−1).
-
(iv)
The identified distinct vibrational peaks within the range 1200–1500 cm−1 are related to vibrations of triangular (BO3) groups.
-
(v)
The rest vibrational peaks in the near-IR region at about 1640 cm−1, 2850, 2950, and 3450–3560 cm−1 are related to vibrations of OH, water, and BOH.
-
(vi)
Regarding the glasses containing small dopants of Bi2O3, several authors (Bishay and Maghrabi 1969; Stehla et al. 1998; ElBatal 2007; ElBatal et al. 2007, 2020; Ouis et al. 2020) have claimed the Bi-O bending vibrations at (460–479) cm−1 and 840 cm−1 as stretching vibrations of Bi–O (BiO3).
The identified IR spectra of these glasses can be explained on the following basis (Abdel-Kader et al. 1991; Mostafa and El-Egili 1998; ElBatal 2008; Marzouk et al. 2017):
-
(a)
The IR bands are originating from the vibrations of the structural groups within the studied glasses.
-
(b)
The basic host glass consists of (LiF50–P2O5 50 mol%) and hence it is found that the structural groups are (PO4), (PO3F) and possible additional (LiF4) groups beside modifier Li+ ions present in noticeable percent.
-
(c)
The assignment of the identified IR peaks are assumed to be:
-
(i)
The far—IR peaks at 400–450 cm−1 can be correlated to vibrations of (Li+) ions in their characteristic sites.
-
(ii)
Peaks at 700–730 cm−1 can be attributed to stretching vibrations of P–O–P rings and sharing of vibrations of P–F band in (FPO3).
-
(iii)
The peaks at 860–890 cm−1 are assigned to asymmetric stretching P–O–P groups.
-
(iv)
The peaks at 1020–1050 cm−1 and 1130–1140 cm−1 are assigned to asymmetric stretching vibrations of PO2 groups.
-
(v)
The peak at 1250–1275 cm−1 is attributed to P = O modes.
-
(vi)
The peaks at about 1640, 2850 are related to the stretching of POH water vibrations.
-
(vii)
The near IR band located at 3440 cm−1 is correlated to vibrations of OH, water, POH.
The addition of a few dopant percent of Bi2O3 produces some additional peaks which can be related to Bi–O–Bi in octahedral coordinated BiO6 within the range 450–500 cm−1 and possible BiO3 vibrations at 820–840 cm−1.
To confirm the assumption of the formation of (LiF4) group in both studied glasses containing (LiF 50 mol%), we introduce Fig. 7 revealing the vibrational peaks which are expected from (LiF4) groups from the inspection of IR spectrum laboratory crystalline (LiF) used in the preparation of the two systems.
3.2 Optical absorption spectral measurements of the prepared glasses
Figures 8 and 9 illustrate the UV–visible absorption spectra of both two different fluoroborate and fluorophosphate glasses from the two systems LiF-B2O3 and LiF-P2O5 and their dopant samples with Bi2O3(1, 2%). The optical spectra of the two undoped glasses of (LiF-B2O3) and (LiF-P2O5) reveal distinct UV absorption within the range of 200–270 nm.
The glasses doped with Bi2O3 (1,2%) show additional small peaks at 330–350 nm and 400–420 nm for the two systems(LiF-B2O3) and (LiF-P2O5).
The observed distinct UV absorption identified in the optical spectra of the two undoped fluoroborate and fluorophosphate glasses can be proposed to result from unavoidable contaminated traces of ferric ions exist as contaminants within the laboratory chemicals applied for the preparation of the prepared glasses. This hypothesis is based on the following items:
-
(i)
Some glass researchers (Sigel and Ginther 1968; Sigel 1977) early assumed that the appearance of distinct UV absorption bands in the spectra of undoped glasses is due to the contamination of impurities (e.g.Fe3+, Cr6+).
-
(ii)
Duffy (Duffy 1997) in his reference article on charge transfer spectra in glasses assumed that UV absorption peaks generally observed in the optical spectra of glasses are due to two reasons:
-
(iii)
The existence of trace impurities of certain 3d transition metals (e.g. Fe3+, Cr6+) produces distinct UV absorption due to its charge transfer origin.
-
(iv)
Duffy (Duffy 1997) also revealed that some other metal ions (such as Ce3+, Tb3+, U4+), as well as d10s2 ions (Pb2+, Bi3+), absorb radiation through electronic transitions involving orbitals essentially of the metal ion only and such UV spectra generally necessitate higher concentrations of the metal ions (Pb2+, Bi3+).
It is therefore presumed that the strong UV absorption bands observed within the range 200–310 nm in the spectra of the two undoped glasses are correlated to trace Fe3+ ions present as impurities and the observed small peaks within the range 350–430 nm are related to absorption of Bi3+ ions. The same assumptions have been reached by several authors (ElBatal and Nucl. 2007; ElBatal et al. 2007, 2020; Ouis et al. 2020; Marzouk et al. 2017b; Fayad et al. 2022).
3.3 Photoluminescence spectral data
Figure 10 illustrates the PL spectra of the base (LiF-B2O3) glass and two Bi2O3–doped glasses. The emission spectra are observed to extend from 375 to about 700 nm with distinct peaks at 415, 465, 505, 525, 560, and 620 nm. The intensity of the peaks increases with the increase of Bi2O3 content. Also, the intensities of the emission peaks decrease with the increase of the wavelength. The excitation peaks reveal two distinct peaks at 266 and 280 nm with the increase of the intensity with increasing wavelength.
Figure 11 reveals the PL spectra of the undoped and Bi2O3—doped within fluoro-phosphate glasses (LiF-P2O5). The emission spectra show three broad bands with peaks at 410, 562, and 557 nm, and the intensities of the first two are nearer while the third peak is lower in intensity. The excitation spectra show two connected bands with peaks at 253 and 279 nm and the latter is more intense than the first peak.
The results shown in Figs. 10 and 11 reveal that the emission spectral peaks identified vary with the type of studied host glass. They show extended emission from about 375 nm to 700 nm in both (LiF-B2O3) and (LiF-P2O5) host glasses. However, in host borate glass, 6 peaks could be identified while in host phosphate glass only three peaks are observed. The observed excitation peaks within the two host glasses are two but show some variations in their positions, the borate peaks are identified at 266 and 280 nm while the phosphate host glass's peaks are at 253 and 279 nm. The previous results can be explained on the following basis (Singh and Karmakar 2012; Tabaza et al. 2014; Singh et al. 2010; Marzouk et al. 2014; Marzouk 2012);
-
(i)
It is agreed that the luminescence properties in glasses are generally influenced and affected by the presence of heavy metal and highly polarized Bi3+ and Pb2+ ions, besides the detailed composition and the structural groupings and their arrangements.
-
(ii)
The changes in the number and position of the PL peaks can be attributed to different sites for Bi3+ ions in different host glasses.
-
(iii)
The previous postulations agreed with the identified IR and optical spectra for the same glasses. IR spectra of the borate glasses reveal highly intense vibrational bands than phosphate glasses because the boron atom is the lightest glass-forming element and is highly affected to easily shows distinct vibrational peaks.
On the other hand, the optical spectra of phosphate glasses reveal higher intensities of the UV and near-visible bands and this latter visible band is related to vibrations of Bi3+ ions
-
(iv)
The same assumptions can be related to the identified changes in the excitation data which refer to the action of host glass on the intensity of the bands.
-
(v)
In a recent publication from Xiang et al. (Wu et al. 2020) on the photoluminescence spectra of bismuth in Na2O-Y2O3. B2O3 phosphor candidate which refer to the of high–quality white LED lighting applications that can be effectively excited in the near UV region (350–400 nm) and show no reabsorption of Bi3+ ions in the visible light region. They have carried out their studies with the ranges (0.05, 0.125, 0.25, 0.5, and 1%) of bismuth ions. They refer that Bi3+ has a d10 s2 electronic layer structure, the electronic configuration is [Xe] 4F14 5d10 6s2, and its luminescence characteristics are closely related to the chemical composition of the host material. They added that the spectrum of Bi3+ is due to the excitation 1S0 and the excited state energy levels are 1P1, 3P2, 3P1, and 1P0 in order of energy.
They concluded that at room temperature, the lowest absorption band of Bi3+ luminescence material is due to the 1S0 → 3P1 transition, while the emission band corresponds to the 3p1 →1s0 transition.
In conclusion and based on previous considerations, the distinct changes identified can be related to the difference between the luminescence spectra of bismuth ions in host borate glass and that in host phosphate glass which can be related to change of the sites of bismuth ions. Optical spectra indicate that the bismuth is in the trivalent state from its characteristic near-visible band.
The CIE1931 chromaticity diagram is used to detect the color emitted by a light source using three dimensionless quantities x̄(λ), ȳ(λ) and z̄(λ). The tristimulus values for a non-monochromatic light source with spectral relative power P (λ) are estimated by Malacara (2011); Mortimer and Varley 2011):
where X, Y, and Z are the tristimulus values to specify the three primary colors of light, red, green, and blue that are required to match the color P(λ), and from the tristimulus values, the color chromaticity coordinates x and y can be determined using the following expressions (Malacara 2011; Mortimer and Varley 2011);
According to Fig. 12, the color coordinates are represented and extended in the blue area under the UV excitations 280 nm for both fluorophosphate and fluoroborate samples. The actual values of CIE color coordinates are (0.198, 0.315) for bismuth borate and (0.217, 0.262) for bismuth phosphate. The observed luminescent blue color is correlated to the presence of heavy metal and highly polarized Bi3+ and Pb2+ ions in addition to the building structure and attached of trivalent bismuth ions in different sites of the host glasses (Denker et al. 2010).
3.4 Thermal expansion data
Figure 13 illustrates the thermal expansion curves of the undoped LiF-B2O3 glass and derived Bi2O3—doped samples. The results indicate that the dilatometric transition temperature and softening temperature for the undoped glass are 392 °C and 430 °C and these parameters decrease with the addition of Bi2O3 to a noticeable degree but the two Bi2O3—doped samples are nearer in their thermal parameters.
Figure 14 reveals the thermal expansion curves of the undoped and Bi2O3—doped LiF-P2O5 glasses. The overall behavior is generally similar to that identified with (LiF-B2O3) glasses but with slightly lower temperatures.
The collected thermal expansion results can be interpreted on the following parameters (Holloway 1973; Rawson 1980; EzzElDin and ElBatal 1993; Shelby 2005).
-
(i)
The glass generally expands upon heating like normal solids except few cases. Normal expansion is occurred as a result of the increased extent of the atomic vibrations of the constituents which may include variation in the bond angles and/or bond lengths within the structural groups.
-
(ii)
The continuous measured increase in the thermal expansion coefficient with temperature is expected and attributed to the collected energy with the temperature rise.
-
(iii)
The observed lowering of the dilatometric softening temperature with the introduction and increase of Bi2O3 is correlated with the known lower melting temperature of Bi2O3 and the housing of Bi3+ in modifying positions within the parent of dopping.
4 Conclusion
Undoped binary lithium fluoroborate (LiF-B2O3) and lithium fluorophosphate (liF-P2O5) glasses together with samples containing Bi2O3– doped (1 or 2%) were prepared by the normal melting—annealing method. Multiple characterizations of the prepared glasses were carried out through analyzing their optical (UV–visible), FT infrared, and photoluminescence spectra. Optical spectra of the two undoped (liF-B2O3) and (LiF-P2O5) glasses reveal only distinct UV absorption which is correlated with unavoidable traces of Fe3+ ions present as impurities within the chemicals used for the preparation of the glasses. The Bi2O3—doped samples reveal additional near-visible bands which are related to the specific absorption of Bi3+ ions. FTIR spectra of the studied glasses reveal vibrational bands which are characteristic for the main structural–forming units (BO3, BO3F)for the borate samples and (PO4, PO3F) groups in the spectra of the (LiF-P2O5) samples. Due to the existence of an unfamiliar high percent of LiF with (50 mol%) in the two host studied glasses, it is assumed that additional vibrational bands of (LiF4) groups are to be expected in the collected IR spectra. Photoluminescence spectral data indicate the appearance of emission and excitation spectra in connection with the Bi3+ ions but are highly defined in host borate than host phosphate glass. This behavior is related to the possible presence of Bi3+ ions in different sites. Thermal expansion data indicate that the introduction of Bi2O3 decreases the transformation and dilatometric softening temperatures due to the low melting of Bi2O3 and the housing of bismuth ions in modifying positions.
Data availability
The authors stated and declare that all data is exist and is available.
References
Abdel-Kader, A., Higazy, A.A., Elkholy, M.M.: Compositional dependence of infrared absorption spectra studies for TeO2-P2O5 and TeO2-P2O5-Bi2O3 glasses. J. Mater. Sci.: Mater. Electronics 2(3), 157–163 (1991)
Bishay, A., Maghrabi, C.: Properties of bismuth glasses in relation to structure. Phys. Chem. Glasses 10, 1 (1969)
Denker, B.I., Galagan, B.I., Osiko, V.V., Shulman, I.L., Sverchkov, S.E., Dianov, E.M.: Factors affecting the formation of near infrared-emitting optical centers in Bi-doped glasses. Appl. Phys. B 98, 455–458 (2010)
Donald, I. W.: in Encylopedia Sci. Eng. vol 3 D.1689 (1993).
Doweidar, H., El-Damarawi, G., Abdelghany, M.: Structure and properties of CaF2–B2O3 glasses. J. Mater. Sci. 47, 4028–4035 (2012)
Duffy, J.A.: Charge transfer spectra of metal ions in glass. Phys. Chem. Glasses 38, 289–292 (1997)
Dumbaugh, W.H., Lapp, J.C.: Heavy-metal oxide glasses. J. Amer. Ceram Soc. 75, 2315–2326 (1992)
Ehrt, D.: Review: phosphate and fluoride phosphate optical glasses—properties, structure and applications. Phys. Chem. Glasses: J. Glass Sci. Technol. B 56(6), 217–234 (2015)
El Batal, F.H.: Gamma ray interaction with bismuth silicate glasses. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Int. Mater. Atoms 254(2), 243–253 (2007)
ElBatal, F.H.: Gamma ray interaction with copper-doped sodium phosphate glasses. J. Mater. Sci 43, 1070–1079 (2008)
ElBatal, F.H., Ouis, M.A.: UV–visible and infrared absorption spectroscopic studies of gamma irradiated tungsten-doped lithium phosphate glasses. Physica B: Condensed Matter 406(22), 4175–4182 (2011)
ElBatal, F.H., Marzouk, S.Y., Nada, N., Desouky, S.M.: Gamma-ray interaction with copper-doped bismuth–borate glasses. Physica B 391, 88–97 (2007)
ElBatal, H.A., Abou-Hussein, E.M., El-Alaily, N.A., AzzElDin, F.M.: Effect of different 3d transition metal oxides on some physical properties of γ-Irradiated Bi2O3–B2O3 glasses: a comparative study. J. Non-Cryst. Solids 528, 19733 (2020)
EzzElDin, F.M., ElBatal, H.A.: Radiation effects on thermal expansion of some ternary alkali-silicate glasses. J. Non-Cryst. Solids 152, 195–200 (1993)
Fayad, A. M., ElBatal, F. H. , Marzouk, M. A.: Spectral and Luminescence Properties of Manganese-Ion-Doped Binary Lithium Fluoride-Phosphate (50 LiF–50 P2O5) Glasses: Impact of Gamma Irradiation, J. Electronic. Materials, 51, pages321–330 (2022).
Gressler, C.A., Shelby, J.E.: Lead fluoroborate glasses. J. Appl. Phys. 64, 4450 (1988)
Holloway, D. G.: The physical properties of Glass, Wykham, London, 36 (1973)
Kamitsos, E.I.: Infrared studies of borate glasses. Phys. Chem. Glasses 44, 79–87 (2003)
Kamitsos, E.I., Patsis, A.P., Karakassides, M.A., Chryssikos, G.D.: Infrared reflectance spectra of lithium borate glasses. J. Non-Cryst. Solids 126(1–2), 52–67 (1990)
Krogh-Moe, J.: Structural interpretation of melting point depression in the sodium borate system, Phys.Chem.Glasses, 3, 101–110(1962); Interpretation of infrared spectra of boron oxide and alkali borate glasses, Phys.Chem.Glasses, 6, 46–54(1965); The structure of vitreous and liquid boron oxide, J.Non-Cryst.Solids, 1(1969) 269–284.
Kondakis, I., Varsamis, C.P.E., Kamitsos, E.I., Moncke, D., Ehrt, D.: Structure and properties of mixed strontium-manganese metaphosphate glasses. J. Phys. Chem. C 114, 9125–9138 (2010)
Lucas, J.: Review: Fluoride glasses. J. Mater. Sci. 24, 1–13 (1989)
Malacara, D.: Color vision and colorimetry; theory and applications,2nd ed., SPIE press, Bellingham, Washington, USA (2011).
Marzouk, M.A.: Optical characterization of some rare earth ions doped bismuth borate glasses and effect of gamma irradiation. J. Mol. Struct. 1019, 80–90 (2012)
Marzouk, M. A., Ibrahim, S., Hamdy, Y. M.: Luminescence efficiency growth in wide band gap semiconducting Bi2O3 doped Cd0.4Pb0.1B0.5 glasses and effect of γ-irradiation, J. Mol. Struct., 1076, 576–582 (2014)
Marzouk, M.A., Hamdy, Y.M., Elbatal, H.A.: Photoluminescence and spectralperformance of manganese ions in zinc phosphate and barium phosphatehost glasses. J. of Non-Cryst. Solids 458, 1–14 (2017a)
Marzouk, M.A., ElBatal, F.H., Elbadry, K.M., ElBatal, H.A.: Optical, structural and thermal properties of sodium metaphosphate glasses containing Bi2O3 with interactions of gamma rays. Spectrochimica Act A 171, 454–460 (2017b)
Möncke, D., Ehrt, D.: Review on the structural analysis of fluoride-phosphate and fluorophosphates glasses. J. Non-Cryst. Solids X3, 100026 (2019)
Mortimer, R.J., Varley, T.S.: Quantification of colour stimuli through the calculation of CIE chromaticity coordinates and luminance data for application to in situ colorimetry studies of electrochromic materials. Displays 32, 35–44 (2011)
Mostafa, Y.M., El-Egili, K.: Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 240, 144–153 (1998)
Ouis, M.A., ElBatal, F.H., Azooz, M.A.: FTIR, optical, and thermal studies of cadmium borate glass doped with Bi2O3 and effects of gamma irradiation. J. Australian Ceramic Society 56, 283–290 (2020)
Rawson, H.: Properties ans applications of glass, Glass science and technology, 3, p. 69. Elsevier, Amesterdam (1980)
Shelby, J.E.: Introduction of Glass Science and Technology, 2nd edn. Royal Society of Chemistry, UK (2005)
Shelby, J.E., Baker, L.D.: Alkali fluoroborate glasses. Phys. Chem. Glasses 39, 23–28 (1998)
Shelby, J.E., Ortolanor, L.: Properties and structure of NaF–Na2O–B2O3 glasses. Phys Chem. Glasses 31, 25–31 (1990)
Sigel Jr., G.H.: in: Treatise on Materials Science and Technology, Eds. M. Tomozawa, R.H.Doremus, vol 12, p.p.3–89, Academic Press, New York (1977).
Sigel, G.H., Jr., Ginther, R.J.: The effect of iron on the ultraviolet absorption of high purity soda-silica glass. Glass Technol. 9, 66–73 (1968)
Singh, S. P., Karmakar, B.: Bismuth oxide and bismuth oxide doped glasses for optical and photonic applications, Bismuth characteristics, Production and applications, Material Science Technologies, Nova, New York, Chapter 9, (2012).
Singh, S.P., Chakradhar, R.P.S., Rao, J.L., Karmakar, B.: EPR, FTIR, optical absorption and photoluminescence studies of Fe2O3 and CeO2 doped ZnO–Bi2O3–B2O3 glasses. J. Alloys Compd. 493, 256–262 (2010)
Stehla, C., Vira, C., Vira, D., Hogan, D., Feller, S.A., Affatigatio, M.A.: Optical and physical properties of bismuth borate glasses related to structure. Phys. Chem. Glasses 39, 83–86 (1998)
Tabaza, W. A. I., Swart, H. C., Kroon, R. E.: Luminescence of Ce doped MgAl2O4 prepared by the combustion method, Physica B, 439, 109–114 (2014) .
Wu, S., Xiong, P., Liu, X., Fu, Y., Liu, Q., Peng, M., Chen, Y., Ma, Z.: Bismuth activated high thermal stability blue-emitting phosphor Na2Y2B2O7: Bi used for near-UV white-light LEDs. J. Mater. Chemistry (c) 8, 16584–16592 (2020)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare no Fund.
Author information
Authors and Affiliations
Contributions
F.H. ElBatal, M.A. Marzouk, H.A. ElBatal, and Y.M. Hamdy contributed to executing data analysis, writing manuscript, drawing figures, and manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ElBatal, F.H., Marzouk, M.A., ElBatal, H.A. et al. Behavior of bismuth ions in the two glassy systems of 50 LiF-50 P2O5 and 50 LiF-50 B2O3 (mol %) assessed by optical, FTIR and photoluminescence spectra in addition to thermal expansion properties. Opt Quant Electron 55, 217 (2023). https://doi.org/10.1007/s11082-022-04475-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-022-04475-0