Abstract
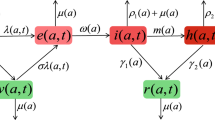
This work explores the dynamics of an epidemic considering an SIVIS (susceptible-infected-vaccinated-infected-susceptible) epidemiological model, accounting for heterogeneous susceptibility, governmental interventions, social behavioral dynamics and public reactions in both of autonomous and nonautonomous aspects. The study frames the system as an optimal control problem, considering time-dependent control strategies for strength of social behavior of public and pharmaceutical treatments. The emergence of a coexistence steady state is analyzed based on the basic reproduction number. The impact of model parameters on disease propagation is assessed through sensitivity analysis. Transcritical bifurcation-induced stability alteration is explored, and numerical simulations illustrate theoretical findings. The proposed system investigates the dynamical behavior in case of periodic transmission rate. It vividly highlights the profound impact of factors such as vaccination rates, frequency and amplitude of transmission on the enduring and evolving dynamic patterns exhibited by the disease.















Similar content being viewed by others
Data availability
The data used to support the findings of the study are available within the article.
References
Bernoulli, D., Chapelle, D.: Essai d’une nouvelle analyse de la mortalité causée par la petite vérole, et des avantages de l’inoculation pour la prévenir 1–45, hal–04100467 (2023)
Dietz, K., Heesterbeek, J.: Daniel Bernoulli’s epidemiological model revisited. Math. Biosci. 180(1–2), 1–21 (2002)
Kermack, W.O., McKendrick, A.G.: A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Charac. 115(772), 700–721 (1927). https://doi.org/10.1098/rspa.1927.0118
Kermack, W.O., McKendrick, A.G.: Contributions to the mathematical theory of epidemics. II. The problem of endemicity. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Charac. 138(834), 55–83 (1932)
Zhan, X.-X., Liu, C., Zhou, G., Zhang, Z.-K., Sun, G.-Q., Zhu, J.J., Jin, Z.: Coupling dynamics of epidemic spreading and information diffusion on complex networks. Appl. Math. Comput. 332, 437–448 (2018). https://doi.org/10.1016/j.amc.2018.03.050
Wang, Y., Wei, Z., Cao, J.: Epidemic dynamics of influenza-like diseases spreading in complex networks. Nonlinear Dyn. 101, 1801–1820 (2020). https://doi.org/10.1007/s11071-020-05867-1
Brauer, F., Castillo-Chavez, C., Feng, Z.: Mathematical Models in Epidemiology, vol. 32. Springer, Berlin (2019)
Martcheva, M.: An Introduction to Mathematical Epidemiology, vol. 61. Springer, Berlin (2015)
Vynnycky, E., White, R.: An Introduction to Infectious Disease Modelling. OUP, Oxford (2010)
Lin, Q., Zhao, S., Gao, D., Lou, Y., Yang, S., Musa, S.S., Wang, M.H., Cai, Y., Wang, W., Yang, L., et al.:: A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan. China with individual reaction and governmental action. Int. Journal . Infect. Dis. 93(2020), 211–216 (2019)
Kwuimy, C., Nazari, F., Jiao, X., Rohani, P., Nataraj, C.: Nonlinear dynamic analysis of an epidemiological model for COVID-19 including public behavior and government action. Nonlinear Dyn. 101, 1545–1559 (2020)
He, D., Dushoff, J., Day, T., Ma, J., Earn, D.J.: Inferring the causes of the three waves of the 1918 influenza pandemic in England and Wales. Proc. R. Soc. B Biol. Sci. 280(1766), 20131345 (2013)
Saha, S., Dutta, P., Samanta, G.: Dynamical behavior of SIRS model incorporating government action and public response in presence of deterministic and fluctuating environments. Chaos Solitons Fract. 164, 112643 (2022). https://doi.org/10.1016/j.chaos.2022.112643
Das, M., Samanta, G., De la Sen, M.: A fractional order model to study the effectiveness of government measures and public behaviours in COVID-19 pandemic. Mathematics 10(16), 3020 (2022)
Zhang, Z.-K., Liu, C., Zhan, X.-X., Lu, X., Zhang, C.-X., Zhang, Y.-C.: Dynamics of information diffusion and its applications on complex networks. Phys. Rep. 651, 1–34 (2016). https://doi.org/10.1016/j.physrep.2016.07.002
Pagliara, R., Dey, B., Leonard, N.E.: Bistability and resurgent epidemics in reinfection models. IEEE Control Syst. Lett. 2(2), 290–295 (2018). https://doi.org/10.1109/LCSYS.2018.2832063
Miller, J.C.: Epidemic size and probability in populations with heterogeneous infectivity and susceptibility. Phys. Rev. E 76(1), 010101 (2007). https://doi.org/10.1103/PhysRevE.76.010101
Katriel, G.: The size of epidemics in populations with heterogeneous susceptibility. J. Math. Biol. 65(2), 237–262 (2012). https://doi.org/10.1007/s00285-011-0460-2
Nakata, Y., Omori, R.: Epidemic dynamics with a time-varying susceptibility due to repeated infections. J. Biol. Dyn. 13(1), 567–585 (2019). https://doi.org/10.1080/17513758.2019.1643043
Zhang, X., Fu, J., Hua, S., Liang, H., Zhang, Z.-K.: Complexity of government response to COVID-19 pandemic: a perspective of coupled dynamics on information heterogeneity and epidemic outbreak. Nonlinear Dyn. 1–20 (2023). https://doi.org/10.1007/s11071-023-08427-5
Gao, S., Chen, L., Nieto, J.J., Torres, A.: Analysis of a delayed epidemic model with pulse vaccination and saturation incidence. Vaccine 24(35–36), 6037–6045 (2006)
Nandhini, M., Lavanya, R., Nieto, J.J.: A fractional COVID-19 model with efficacy of vaccination. Axioms 11(9), 446 (2022)
Arino, J., McCluskey, C.C., van den Driessche, P.: Global results for an epidemic model with vaccination that exhibits backward bifurcation. SIAM J. Appl. Math. 64(1), 260–276 (2003)
Trawicki, M.B.: Deterministic SEIRS epidemic model for modeling vital dynamics, vaccinations, and temporary immunity. Mathematics 5(1), 7 (2017)
Cao, B., Shan, M., Zhang, Q., Wang, W.: A stochastic SIS epidemic model with vaccination. Physica A 486, 127–143 (2017)
Saha, S., Samanta, G., Nieto, J.J.: Impact of optimal vaccination and social distancing on COVID-19 pandemic. Math. Comput. Simul. 200, 285–314 (2022)
Turkyilmazoglu, M.: An extended epidemic model with vaccination: weak-immune SIRVI. Physica A 598, 127429 (2022)
Zaman, G., Kang, Y.H., Jung, I.H.: Stability analysis and optimal vaccination of an SIR epidemic model. Biosystems 93(3), 240–249 (2008)
Sharma, S., Samanta, G.P.: Stability analysis and optimal control of an epidemic model with vaccination. Int. J. Biomath. 8(03), 1550030 (2015). https://doi.org/10.1142/S1793524515500308
Organization, W.H., et al.: Review of latest available evidence on potential transmission of avian influenza (H5N1) through water and sewage and ways to reduce the risks to human health. Technical reports, World Health Organization (2006)
Weber, T.P., Stilianakis, N.I.: Migratory birds, the H5N1 influenza virus and the scientific method. Virol. J. 5(1), 1–3 (2008)
Prosser, D.J., Cui, P., Takekawa, J.Y., Tang, M., Hou, Y., Collins, B.M., Yan, B., Hill, N.J., Li, T., Li, Y., et al.: Wild bird migration across the Qinghai-Tibetan plateau: a transmission route for highly pathogenic H5N1. PLOS ONE 6(3), e17622 (2011)
Greer, M., Saha, R., Gogliettino, A., Yu, C., Zollo-Venecek, K.: Emergence of oscillations in a simple epidemic model with demographic data. R. Soc. Open Sci. 7(1), 191187 (2020)
Buonomo, B., Chitnis, N., d’Onofrio, A.: Seasonality in epidemic models: a literature review. Ricerche Mat. 67, 7–25 (2018)
Kambali, P.N., Abbasi, A., Nataraj, C.: Nonlinear dynamic epidemiological analysis of effects of vaccination and dynamic transmission on COVID-19. Nonlinear Dyn. 111(1), 951–963 (2023)
Nazarimehr, F., Pham, V.-T., Kapitaniak, T.: Prediction of bifurcations by varying critical parameters of COVID-19. Nonlinear Dyn. 101, 1681–1692 (2020)
He, S., Peng, Y., Sun, K.: SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dyn. 101, 1667–1680 (2020)
Hale, J.K.: Theory of Functional Differential Equations. Springer, New York (1977)
Van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180(1–2), 29–48 (2002)
Arriola, L., Hyman, J.: Lecture notes, forward and adjoint sensitivity analysis: with applications in Dynamical Systems. In: Linear Algebra and Optimisation. Mathematical and Theoretical Biology Institute (Summer) (2005)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1(2), 361–404 (2004). https://doi.org/10.3934/mbe.2004.1.361
Martcheva, M.: An evolutionary model of influenza A with drift and shift. J. Biol. Dyn. 6(2), 299–332 (2012)
Tuncer, N., Martcheva, M.: Modeling seasonality in avian influenza H5N1. J. Biol. Syst. 21(04), 1340004 (2013)
Ma, J., Ma, Z.: Epidemic threshold conditions for seasonally forced SEIR models. Math. Biosci. Eng. 3(1), 161 (2006)
Greenhalgh, D., Moneim, I.: SIRS epidemic model and simulations using different types of seasonal contact rate. Syst. Anal. Model. Simul. 43(5), 573–600 (2003)
Kassa, S.M., Ouhinou, A.: The impact of self-protective measures in the optimal interventions for controlling infectious diseases of human population. J. Math. Biol. 70(1–2), 213–236 (2015). https://doi.org/10.1007/s00285-014-0761-3
Gaff, H., Schaefer, E.: Optimal control applied to vaccination and treatment strategies for various epidemiological models. Math. Biosci. Eng. 6(3), 469–492 (2009). https://doi.org/10.3934/mbe.2009.6.469
Kirk, D.E.: Optimal Control Theory: An Introduction. Courier Corporation, North Chelmsford (2004)
Disease Control Programmes (NHM), https://main.mohfw.gov.in/sites/default/files/05%20ChapterAN2018-19.pdf
Coddington, A., Levinson, N.: Theory of ordinary differential equations. In: International Series in Pure and Applied Mathematics. Tata McGraw-Hill Companies (1955)
Fleming, W., Rishel, R.: Deterministic and Stochastic Optimal Control, vol. 1. Springer, New York (1975)
Pontryagin, L.S.: Mathematical Theory of Optimal Processes. CRC Press, Boca Raton (1987)
Acknowledgements
The authors are grateful to the learned reviewers and Prof. Jun Ma (Editor) for their careful reading, valuable comments and helpful suggestions, which have helped them to improve the presentation of this work significantly. A part of this research was completed during the visit of G. Samanta to the CITMAga (Galician Centre for Mathematical Research and Technology) and the University of Santiago de Compostela in January 2023. The research of J.J. Nieto has been partially supported by the Agencia Estatal de Investigacion (AEI) of Spain, cofinanced by the European Fund for Regional Development (FEDER) corresponding to the 2014–2020 multiyear financial framework, project PID2020-113275GB-I00.
Funding
The research of J.J. Nieto has been partially supported by the Agencia Estatal de Investigacion (AEI) of Spain, cofinanced by the European Fund for Regional Development (FEDER) corresponding to the 2014-2020 multiyear financial framework, project PID2020-113275GB-I00.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A
1.1 A.1 Reproduction number
Suppose that, \(x\equiv (I,V)\). Then we have:
Here, \({\mathfrak {F}}(x)\) consists of the compartment in which infection is introduced first and \(\nu (x)\) contains rest of the terms. Then, at \(\displaystyle E_{0}(S_{10},S_{20},0,V_0)\), we have
Reproduction number is the spectral radius of next-generation matrix. Now, \(FV^{-1}\) is the next-generation matrix whose spectral radius is denoted as:
Appendix B
1.1 B.1 Existence of optimal control functions
Here, we describe the existence of optimal control measures with a minimized cost function in a finite time interval.
Proof of Theorem 8.1
Model (8.2) assumes \(N=S_1+S_2+I+V\) be the total population. So,
where N(0) represents the overall population at initial state. As \(\displaystyle t\rightarrow \infty ,\ 0<N(t)\le \frac{\Pi }{\mu }+\epsilon , ~ \mathrm{for ~ any ~} \epsilon > 0.\)
Thus, when control factors are present, the solution of model (8.2) remains bounded, and the functions on the right side of system (8.2) are Lipschitz continuous within \(\Xi \). Thus, according to the Picard–Lindelöf theorem, optimal control model system (8.2) exhibits nontrivial solutions within \(\Xi \) [50]. Moreover, the control elements are confined within a closed and convex set \(\Xi \). Each equation system (8.2) can be expressed linearly using D and \(\gamma \), with coefficients dependent on time and state variables. Moreover, the control variables are of second order, leading to the integrand \(L(S_1,S_2,I,V,D,\gamma )\) being a convex function on the solution set \(\Xi \).
Let us take, \({\overline{g}} = \min (g_{2},g_{3})>0\) and \(h(D,\gamma )={\overline{g}}(D^{k}+\gamma ^{2})\). Hence, \(h(D,\gamma )\) is a continuous function, and \(L(S_1,S_2,I,V,D,\gamma )\ge h(D,\gamma )\). Furthermore, \(||(D,\gamma )||^{-1}h(D,\gamma )\rightarrow \infty \) for \(||(D,\gamma )||\rightarrow \infty .\) Therefore, using the outcomes of [47, 51], it can be stated that optimal control interventions \(D^{*}\) and \(\gamma ^{*}\) exist such that \(Z(D^{*},\gamma ^{*})=\min [Z(D,\gamma )]\). \(\square \)
1.2 B.2 Characterization of control interventions
The optimal control strategies are derived through the utilization of Pontryagin’s principle [51, 52]. Now, the Hamiltonian function is given by:
Here \(l=\left( l_{1},l_{2},l_{3},l_4\right) \) indicates adjoint variables. Our primary concern is to minimize Hamiltonian H by utilizing Pontryagin’s principle so that a minimal cost function is obtained.
Proof of Theorem 8.2
Consider \(D^{*}\) and \(\gamma ^{*}\) be the applied optimal control along with the corresponding optimal state variables are \(S_1^{*}, S_2^*, I^{*}\) and \(V^{*}\) of (8.2) which minimize the cost functional Z defined in (8.1). Therefore, we have adjoint variables \(l_{i}\) for \(i=1,2,3,4\), satisfying the canonical equations:
So, we have
with the transversality conditions \(l_{i}(T_{f})=0\), for \(i=1,2,3,4\).
So, \(\displaystyle D^{*}{=} \frac{\left[ \frac{(1{-}\alpha )I^*}{g_{2}}\left\{ (l_{3}{-}l_{1})\theta _1 S_1^* {+} (l_{3}{-}l_{2})\theta _2 S_2^* \right\} \right] ^{\frac{1}{k-1}}}{1{+}\left[ \frac{(1{-}\alpha )I^*}{g_{2}}\left\{ (l_{3}{-}l_{1})\theta _1 S_1^* {+} (l_{3}{-}l_{2})\theta _2 S_2^* \right\} \right] ^{\frac{1}{k-1}}}\)
and \(\displaystyle \gamma ^{*}{=}\frac{ I^{*}}{2g_{3}}\left\{ \left( l_{4}-l_{1}\right) \beta _1+\left( l_{4}-l_{2}\right) \beta _2 -\left( l_{4}-l_{3}\right) \right\} \).
In \(\Xi ,\) we have
which is equivalent as (8.4). \(\square \)
1.3 B.3 Optimal system
The optimal system which includes the optimal control measures \(D^{*}\) and \(\gamma ^{*}\) and minimizing the Hamiltonian \(H^{*}\) at \((S_1^{*},S_2^{*}, I^{*}, V^{*}, l_{1}, l_{2}, l_{3},l_4)\) is
with non-negative initial conditions \(S_1^{*}(0)>0,\ S_2^{*}(0)>0, \ I^{*}(0)\ge 0,\ V^{*}(0) \ge 0\), and the corresponding adjoint system is:
with transversality conditions \(l_{i}(T_{f})=0\), for \(i=1,2,3,4\) and the control interventions \(D^{*},\ \gamma ^{*}\) are the same as in (8.4).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dutta, P., Samanta, G. & Nieto, J.J. Periodic transmission and vaccination effects in epidemic dynamics: a study using the SIVIS model. Nonlinear Dyn 112, 2381–2409 (2024). https://doi.org/10.1007/s11071-023-09157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-09157-4