Abstract
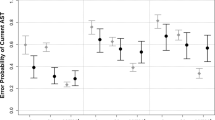
Persons with Mild Cognitive Impairment (MCI) are at high Alzheimer’s Disease (AD) risk but the development of sensitive measures to assess subtle cognitive decline in this population poses a major challenge for clinicians and researchers. Eye movement monitoring is a non-invasive, sensitive way to assess subtle cognitive processes in clinical populations. We conducted a critical review and a meta-analysis of the literature on pro and antisaccade paradigm in AD/MCI. The meta-analysis included 20 studies, all of which used the prosaccade paradigm and 13 of which studied the antisaccade paradigm as well. Our meta-analysis showed that AD but not MCI patients showed longer prosaccade latencies when compared to controls. While antisaccade latencies did not differentiate between patients from controls, antisaccade error rate were significantly increased among patients in comparison to controls in over 87% of the studies. These findings highlight antisaccade error rate as a reliable tool to distinguish inhibition abilities between AD/MCI and healthy older persons.



Similar content being viewed by others
References
Abel, L. A., Unverzagt, F., & Yee, R. D. (2002). Effects of stimulus predictability and interstimulus gap on saccades in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 13(4), 235–243.
Alichniewicz, K. K., Brunner, F., Klünemann, H. H., & Greenlee, M. W. (2013). Neural correlates of saccadic inhibition in healthy elderly and patients with amnestic mild cognitive impairment. Frontiers in Psychology, 4, 467.
Alzheimer’s Association. (2015). 2015 Alzheimer's disease facts and figures. Alzheimer's & Dementia, 11(3), 332–384.
Anderson, T. J., & MacAskill, M. R. (2013). Eye movements in patients with neurodegenerative disorders. Nature Reviews Neurology, 9(2), 74–85.
Bélanger, S., Belleville, S., & Gauthier, S. (2010). Inhibition impairments in Alzheimer's disease, mild cognitive impairment and healthy aging: Effect of congruency proportion in a Stroop task. Neuropsychologia, 48(2), 581–590.
Biscaldi, M., Fischer, B., & Hartnegg, K. (2000). Voluntary saccadic control in dyslexia. Perception, 29(5), 509–521.
Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to meta-analysis. Chichester, UK: Wiley.
Boxer, A. L., Garbutt, S., Rankin, K. P., Hellmuth, J., Neuhaus, J., Miller, B. L., et al. (2006). Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. Journal of Neuroscience, 26(23), 6354–6363.
Boxer, A. L., Garbutt, S., Seeley, W. W., Jafari, A., Heuer, H. W., Mirsky, J., et al. (2012). Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer disease. Archives of Neurology, 69(4), 509–517.
Bundesen, C. (1990). A theory of visual attention. Psychological Review, 97(4), 523.
Bylsma, F. W., Rasmusson, D. X., Rebok, G. W., Keyl, P. M., Tune, L., & Brandt, J. (1995). Changes in visual fixation and saccadic eye movements in Alzheimer's disease. International Journal of Psychophysiology, 19(1), 33–40.
Crawford, T. J., Devereaux, A., Higham, S., & Kelly, C. (2015). The disengagement of visual attention in Alzheimer's disease: A longitudinal eye-tracking study. Frontiers in Aging Neuroscience, 7, 118. https://doi.org/10.3389/fnagi.2015.00118.
Crawford, T. J., Higham, S., Mayes, J., Dale, M., Shaunak, S., & Lekwuwa, G. (2013). The role of working memory and attentional disengagement on inhibitory control: effects of aging and Alzheimer's disease. Age, 35(5), 1637–1650.
Crawford, T. J., Higham, S., Renvoize, T., Patel, J., Dale, M., Suriya, A., et al. (2005). Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer’s disease. Biological Psychiatry, 57(9), 1052–1060.
Crutcher, M. D., Calhoun-Haney, R., Manzanares, C. M., Lah, J. J., Levey, A. I., & Zola, S. M. (2009). Eye tracking during a visual paired comparison task as a predictor of early dementia. American Journal of Alzheimer's Disease and Other Dementias, 24(3), 258–266.
Currie, J., Ramsden, B., McArthur, C., & Maruff, P. (1991). Validation of a clinical antisaccadic eye movement test in the assessment of dementia. Archives of Neurology, 48(6), 644–648.
Deubel, H., & Schneider, W. X. (2003). Delayed saccades, but not delayed manual aiming movements, require visual attention shifts. Annals of the New York Academy of Sciences, 1004(1), 289–296.
Eenshuistra, R. M., Ridderinkhof, K. R., & van der Molen, M. W. (2004). Age-related changes in antisaccade task performance: Inhibitory control or working-memory engagement? Brain and Cognition, 56(2), 177–188.
Fletcher, W. A., & Sharpe, J. A. (1986). Saccadic eye movement dysfunction in Alzheimer's disease. Annals of Neurology, 20(4), 464–471.
Garbutt, S., Matlin, A., Hellmuth, J., Schenk, A. K., Johnson, J. K., Rosen, H., et al. (2008). Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain, 131(5), 1268–1281.
Gurnani, A. S., & Gavett, B. E. (2017). The differential effects of Alzheimer's Disease and lewy body pathology on cognitive performance: A meta-analysis. [journal article]. Neuropsychology Review, 27(1), 1–17. https://doi.org/10.1007/s11065-016-9334-0.
Hannula, D. E., Althoff, R. R., Warren, D. E., Riggs, L., Cohen, N. J., & Ryan, J. D. (2010). Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Frontiers in Human Neuroscience, 4(166), 52–67.
Hannula, D. E., Ryan, J. D., Tranel, D., & Cohen, N. J. (2007). Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. Journal of Cognitive Neuroscience, 19(10), 1690–1705.
Hershey, L. A., Whicker, L., Abel, L. A., Dell'Osso, L., Traccis, S., & Grossniklaus, D. (1983). Saccadic latency measurements in dementia. Archives of Neurology, 40(9), 592–593.
Heuer, H. W., Mirsky, J. B., Kong, E. L., Dickerson, B. C., Miller, B. L., Kramer, J. H., et al. (2013). Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology, 81(14), 1235–1243.
Higgins, J. P., & Green, S. (2011). Cochrane handbook for systematic reviews of interventions (Vol. 4). UK: John Wiley & Sons.
Hutton, S. B., & Ettinger, U. (2006). The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology, 43(3), 302–313.
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials, 17(1), 1–12.
Jak, A. J., Preis, S. R., Beiser, A. S., Seshadri, S., Wolf, P. A., Bondi, M. W., et al. (2016). Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. Journal of the International Neuropsychological Society, 1-7.
Kalesnykas, R., & Hallett, P. (1994). Retinal eccentricity and the latency of eye saccades. Vision Research, 34(4), 517–531.
Kaufman, L. D., Pratt, J., Levine, B., & Black, S. E. (2010). Antisaccades: A probe into the dorsolateral prefrontal cortex in Alzheimer's disease. A critical review. Journal of Alzheimer's Disease, 19(3), 781–793.
Kaufman, L. D., Pratt, J., Levine, B., & Black, S. E. (2012). Executive deficits detected in mild Alzheimer's disease using the antisaccade task. Brain and Behavior, 2(1), 15–21.
Kent, B., Hvoslef-Eide, M., Saksida, L., & Bussey, T. (2016). The representational–hierarchical view of pattern separation: Not just hippocampus, not just space, not just memory? Neurobiology of Learning and Memory, 129, 99–106.
Lagun, D., Manzanares, C., Zola, S. M., Buffalo, E. A., & Agichtein, E. (2011). Detecting cognitive impairment by eye movement analysis using automatic classification algorithms. Journal of Neuroscience Methods, 201(1), 196–203.
Leigh, R., & Kennard, C. (2004). Using saccades as a research tool in the clinical neurosciences. Brain, 127(3), 460–477.
Mewborn, C. M., Lindbergh, C. A., & Stephen Miller, L. (2017). Cognitive Interventions for cognitively healthy, mildly impaired, and mixed samples of older adults: A systematic review and meta-analysis of randomized-controlled trials. Neuropsychology Review. https://doi.org/10.1007/s11065-11017-19350-11068.
Michie, S., Abraham, C., Whittington, C., McAteer, J., & Gupta, S. (2009). Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychology, 28(6), 690–701.
Mielke, R., Kessler, J., Fink, G., Herholz, K., & Heiss, W.-D. (1995). Dysfunction of visual cortex contributes to disturbed processing of visual information in Alzheimer's disease. International Journal of Neuroscience, 82(1–2), 1–9.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202.
Minshew, N. J., Luna, B., & Sweeney, J. A. (1999). Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology, 52(5), 917–917.
Mitchell, A. J., & Shiri-Feshki, M. (2009). Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica, 119(4), 252–265.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4(1), 1.
Molitor, R. J., Ko, P. C., & Ally, B. A. (2015). Eye movements in Alzheimer's disease. Journal of Alzheimer's Disease, 44(1), 1–12.
Mosimann, U. P., Müri, R. M., Burn, D. J., Felblinger, J., O'Brien, J. T., & McKeith, I. G. (2005). Saccadic eye movement changes in Parkinson's disease dementia and dementia with Lewy bodies. Brain, 128(6), 1267–1276.
Moss, R. A. (2016). A theory on the singular function of the hippocampus: Facilitating the binding of new circuits of cortical columns. AIMS Neuroscience, 3(3), 264–305.
Nakashima, Y., Morita, K., Ishii, Y., Shouji, Y., & Uchimura, N. (2010). Characteristics of exploratory eye movements in elderly people: possibility of early diagnosis of dementia. Psychogeriatrics, 10(3), 124–130.
O’Driscoll, G. A., Dépatie, L., Holahan, A.-L. V., Savion-Lemieux, T., Barr, R. G., Jolicoeur, C., et al. (2005). Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biological Psychiatry, 57(11), 1452–1460.
Okonkwo, O. C., Wadley, V. G., Ball, K., Vance, D. E., & Crowe, M. (2008). Dissociations in visual attention deficits among persons with mild cognitive impairment. Aging Neuropsychol C, 15(4), 492–505.
Peltsch, A., Hemraj, A., Garcia, A., & Munoz, D. (2011). Age-related trends in saccade characteristics among the elderly. Neurobiology of Aging, 32(4), 669–679.
Peltsch, A., Hemraj, A., Garcia, A., & Munoz, D. P. (2014). Saccade deficits in amnestic mild cognitive impairment resemble mild Alzheimer's disease. European Journal of Neuroscience, 39(11), 2000–2013.
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194.
Petersen, R. C., & Bennett, D. (2005). Mild cognitive impairment: is it Alzheimer's disease or not? Journal of Alzheimer's Disease, 7(3), 241–245.
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., & Kokmen, E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308.
Pratt, J., Abrams, R. A., & Chasteen, A. L. (1997). Initiation and inhibition of saccadic eye movements in younger and older adults: An analysis of the gap effect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 52(2), P103-P107.
Pratt, J., Dodd, M., & Welsh, T. (2006). Growing older does not always mean moving slower: examining aging and the saccadic motor system. Journal of Motor Behavior, 38(5), 373–382.
Rosenthal, R. (1991). Meta-analytic procedures for social research (Vol. 6): Sage.
Rösler, A., Mapstone, M. E., Hays, A. K., Mesulam, M., Rademaker, A., Gitelman, D. R., et al. (2000). Alterations of visual search strategy in Alzheimer's disease and aging. Neuropsychology, 14(3), 398–408.
Rüb, U., Del Tredici, K., Schultz, C., Büttner-Ennever, J., & Braak, H. (2001). The premotor region essential for rapid vertical eye movements shows early involvement in Alzheimer's disease-related cytoskeletal pathology. Vision Research, 41(16), 2149–2156.
Ryan, J. D., Althoff, R. R., Whitlow, S., & Cohen, N. J. (2000). Amnesia is a deficit in relational memory. Psychological Science, 11(6), 454–461.
Sánchez-Meca, J., & Marín-Martínez, F. (1997). Homogeneity tests in meta-analysis: A Monte Carlo comparison of statistical power and Type I error. Quality and Quantity, 31(4), 385–399.
Santana, R., Mendiburu, A., & Lozano, J. A. (2015). Multi-view classification of psychiatric conditions based on saccades. Applied Soft Computing, 31, 308–316.
Saslow, M. (1967). Effects of components of displacement-step stimuli upon latency for saccadic eye movement. JOSA, 57(8), 1024–1029.
Scinto, L. F., Daffner, K. R., Castro, L., Weintraub, S., Vavrik, M., & Mesulam, M. M. (1994). Impairment of spatially directed attention in patients with probable Alzheimer's disease as measured by eye movements. Archives of Neurology, 51(7), 682–688.
Seligman, S. C., & Giovannetti, T. (2015). The potential utility of eye movements in the detection and characterization of everyday functional difficulties in mild cognitive impairment. Neuropsychology Review, 25(2), 199–215.
Shafiq-Antonacci, R., Maruff, P., Masters, C., & Currie, J. (2003). Spectrum of saccade system function in Alzheimer disease. Archives of Neurology, 60(9), 1272–1278.
Shakespeare, T., Yong, K., Kaski, D., Schott, J. M., & Crutch, S. (2014). Abnormalities of fixation, saccade, and pursuit in posterior cortical atrophy compared to typical AD. Alzheimer's & Dementia, 10(4), P199.
Silverberg, N. B., Ryan, L. M., Carrillo, M. C., Sperling, R., Petersen, R. C., Posner, H. B., et al. (2011). Assessment of cognition in early dementia. Alzheimer's & Dementia, 7(3), e60–e76.
Smith, E. E., & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657–1661.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia, 7(3), 280–292.
Sperling, R. A., Dickerson, B. C., Pihlajamaki, M., Vannini, P., LaViolette, P. S., Vitolo, O. V., et al. (2010). Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Medicine, 12(1), 27–43.
Thulborn, K. R., Martin, C., & Voyvodic, J. T. (2000). Functional MR imaging using a visually guided saccade paradigm for comparing activation patterns in patients with probable Alzheimer's disease and in cognitively able elderly volunteers. AJNR, 21(3), 524–531.
Tzekov, R., & Mullan, M. (2014). Vision function abnormalities in Alzheimer disease. Survey of Ophthalmology, 59(4), 414–433.
Verheij, S., Muilwijk, D., Pel, J. J., van der Cammen, T. J., Mattace-Raso, F. U., & van der Steen, J. (2012). Visuomotor impairment in early-stage Alzheimer's disease: changes in relative timing of eye and hand movements. Journal of Alzheimer's Disease, 30(1), 131–143.
Vos, S. J., Verhey, F., Frölich, L., Kornhuber, J., Wiltfang, J., Maier, W., et al. (2015). Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain, 138, 1327–1338.
Webb, T. L., Miles, E., & Sheeran, P. (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138(4), 775–808.
Yang, Q., Wang, T., Su, N., Liu, Y., Xiao, S., & Kapoula, Z. (2011). Long latency and high variability in accuracy-speed of prosaccades in Alzheimer’s disease at mild to moderate stage. Dementia and Geriatric Cognitive Disorders Extra, 1(1), 318–329.
Yang, Q., Wang, T., Su, N., Xiao, S., & Kapoula, Z. (2013). Specific saccade deficits in patients with Alzheimer’s disease at mild to moderate stage and in patients with amnestic mild cognitive impairment. Age, 35(4), 1287–1298.
Yeung, L.-K., Ryan, J. D., Cowell, R. A., & Barense, M. D. (2013). Recognition memory impairments caused by false recognition of novel objects. Journal of Experimental Psychology: General, 142(4), 1384–1397.
Yun, J.-Y., Lee, D. Y., Seo, E. H., Choo, I. H., Park, S. Y., Kim, S. G., et al. (2011). Neural correlates of stroop performance in Alzheimer’s disease: a FDG-PET study. Dementia and Geriatric Cognitive Disorders Extra, 1(1), 190–201.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors report no disclosures or conflict of interest.
Rights and permissions
About this article
Cite this article
Kahana Levy, N., Lavidor, M. & Vakil, E. Prosaccade and Antisaccade Paradigms in Persons with Alzheimer’s Disease: A Meta-Analytic Review. Neuropsychol Rev 28, 16–31 (2018). https://doi.org/10.1007/s11065-017-9362-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-017-9362-4