Abstract
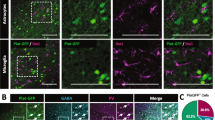
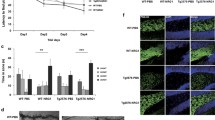
Previously, we reported that epidermal growth factor (EGF) suppresses GABAergic neuronal development in the rodent cortex. Parvalbumin-positive GABAergic neurons (PV neurons) have a unique extracellular structure, perineuronal nets (PNNs). PNNs are formed during the development of PV neurons and are mainly formed from chondroitin sulfate (CS) proteoglycans (CSPGs). We examined the effect of EGF on CSPG production and PNN formation as a potential molecular mechanism for the inhibition of inhibiting GABAergic neuronal development by EGF. In EGF-overexpressing transgenic (EGF-Tg) mice, the number of PNN-positive PV neurons was decreased in the cortex compared with that in wild-type mice, as in our previous report. The amount of CS and neurocan was also lower in the cortex of EGF-Tg mice, with a similar decrease observed in EGF-treated cultured cortical neurons. PD153035, an EGF receptor (ErbB1) kinase inhibitor, prevented those mentioned above excess EGF-induced reduction in PNN. We explored the molecular mechanism underlying the effect of EGF on PNNs using fluorescent substrates for matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinases (ADAMs). EGF increased the enzyme activity of MMPs and ADAMs in cultured neurons. These enzyme activities were also increased in the EGF-Tg mice cortex. GM6001, a broad inhibitor of MMPs and ADAMs, also blocked EGF-induced PNN reductions. Therefore, EGF/EGF receptor signals may regulate PNN formation in the developing cortex.




Similar content being viewed by others
References
Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, Tsuchiya K, Someya T, Kakita A, Takahashi H, Nawa H (2002) Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry 7:673–682. https://doi.org/10.1038/sj.mp.4001081
Piao YS, Iwakura Y, Takei N, Nawa H (2005) Differential distributions of peptides in the epidermal growth factor family and phosphorylation of ErbB 1 receptor in adult rat brain. Neurosci Lett 390:21–24. https://doi.org/10.1016/j.neulet.2005.07.048
Iwakura Y, Nawa H (2013) ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson’s disease. Front Cell Neurosci 7:4. https://doi.org/10.3389/fncel.2013.00004
Iwakura Y, Piao YS, Mizuno M, Takei N, Kakita A, Takahashi H, Nawa H (2005) Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: neurotrophic implication in nigrostriatal neurons. J Neurochem 93:974–983. https://doi.org/10.1111/j.1471-4159.2005.03073.x
Iwakura Y, Zheng Y, Sibilia M, Abe Y, Piao YS, Yokomaku D, Wang R, Ishizuka Y, Takei N, Nawa H (2011) Qualitative and quantitative re-evaluation of epidermal growth factor-ErbB1 action on developing midbrain dopaminergic neurons in vivo and in vitro: target-derived neurotrophic signaling (part 1). J Neurochem 118:45–56. https://doi.org/10.1111/j.1471-4159.2011.07287.x. Epub 2011 May 19
Namba H, Nagano T, Jodo E, Eifuku S, Horie M, Takebayashi H, Iwakura Y, Sotoyama H, Takei N, Nawa H (2017) Epidermal growth factor signals attenuate phenotypic and functional development of neocortical GABA neurons. J Neurochem 142:886–900. https://doi.org/10.1111/jnc.14097
Miyata S, Kitagawa H (2017) Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta Gen Subj 1861:2420–2434. https://doi.org/10.1016/j.bbagen.2017.06.010
Galtrey CM, Fawcett JW (2007) The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54:1–18. https://doi.org/10.1016/j.brainresrev.2006.09.006
Avram S, Shaposhnikov S, Buiu C, Mernea M (2014) Chondroitin sulfate proteoglycans: structure-function relationship with implication in neural development and brain disorders. Biomed Res Int 2014:642798. https://doi.org/10.1155/2014/642798
Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol 13:612–620. https://doi.org/10.1016/j.sbi.2003.09.011
Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S (2009) Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci 30:2053–2063. https://doi.org/10.1111/j.1460-9568.2009.06996.x
Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261. https://doi.org/10.1126/science.1174146
Balmer TS, Carels VM, Frisch JL, Nick TA (2009) Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci 29:12878–12885. https://doi.org/10.1523/jneurosci.2974-09.2009
Miyata S, Komatsu Y, Yoshimura Y, Taya C, Kitagawa H (2012) Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat Neurosci 15(414–22, s1–2). https://doi.org/10.1038/nn.3023
Jodo E, Inaba H, Narihara I, Sotoyama H, Kitayama E, Yabe H, Namba H, Eifuku S, Nawa H (2019) Neonatal exposure to an inflammatory cytokine, epidermal growth factor, results in the deficits of mismatch negativity in rats. Sci Rep 9:7503. https://doi.org/10.1038/s41598-019-43923-y
Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, Nawa H (2003) Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: implication for epidermal growth factor in cognitive development. Mol Psychiatry 8:19–29. https://doi.org/10.1038/sj.mp.4001138
Yukawa T, Iwakura Y, Takei N, Saito M, Watanabe Y, Toyooka K, Igarashi M, Niizato K, Oshima K, Kunii Y, Yabe H, Matsumoto J, Wada A, Hino M, Iritani S, Niwa SI, Takeuchi R, Takahashi H, Kakita A, Someya T, Nawa H (2018) Pathological alterations of chondroitin sulfate moiety in postmortem hippocampus of patients with schizophrenia. Psychiatry Res 270:940–946. https://doi.org/10.1016/j.psychres.2018.10.062
Eda T, Mizuno M, Araki K, Iwakura Y, Namba H, Sotoyama H, Kakita A, Takahashi H, Satoh H, Chan SY, Nawa H (2013) Neurobehavioral deficits of epidermal growth factor-overexpressing transgenic mice: impact on dopamine metabolism. Neurosci Lett 547:21–25. https://doi.org/10.1016/j.neulet.2013.04.055
Mak KK, Chan SY (2003) Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem 278:26120–26126. https://doi.org/10.1074/jbc.M212082200
Iwakura Y, Wang R, Inamura N, Araki K, Higashiyama S, Takei N, Nawa H (2017) Glutamate-dependent ectodomain shedding of neuregulin-1 type II precursors in rat forebrain neurons. PLoS ONE 12:e0174780. https://doi.org/10.1371/journal.pone.0174780
Paxinos G, Franklin KBJ (2013) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. Elsevier/AP, Amsterdam
Iwakura Y, Kawahara-Miki R, Kida S, Sotoyama H, Gabdulkhaev R, Takahashi H, Kunii Y, Hino M, Nagaoka A, Izumi R, Shishido R, Someya T, Yabe H, Kakita A, Nawa H (2022) Elevation of EGR1/zif268, a neural activity marker, in the auditory cortex of patients with Schizophrenia and its animal model. Neurochem Res 47:2715–2727. https://doi.org/10.1007/s11064-022-03599-9
Roghani M, Becherer JD, Moss ML, Atherton RE, Erdjument-Bromage H, Arribas J, Blackburn RK, Weskamp G, Tempst P, Blobel CP (1999) Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J Biol Chem 274:3531–3540. https://doi.org/10.1074/jbc.274.6.3531
Moss ML, Rasmussen FH (2007) Fluorescent substrates for the proteinases ADAM17, ADAM10, ADAM8, and ADAM12 useful for high-throughput inhibitor screening. Anal Biochem 366:144-8. doi: S0003-2697(07)00277-1 [pii]. https://doi.org/10.1016/j.ab.2007.04.043.
Iwakura Y, Wang R, Abe Y, Piao YS, Shishido Y, Higashiyama S, Takei N, Nawa H (2011) Dopamine-dependent ectodomain shedding and release of epidermal growth factor in developing striatum: target-derived neurotrophic signaling (part 2). J Neurochem 118:57–68. https://doi.org/10.1111/j.1471-4159.2011.07295.x
Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L (1998) Perineuronal nets: past and present. Trends Neurosci 21:510–515. https://doi.org/10.1016/s0166-2236(98)01298-3
Hensch TK (2005) Critical period mechanisms in developing visual cortex. Curr Top Dev Biol 69:215–237. https://doi.org/10.1016/s0070-2153(05)69008-4
Kusche-Gullberg M, Kjellén L (2003) Sulfotransferases in glycosaminoglycan biosynthesis. Curr Opin Struct Biol 13:605–611. https://doi.org/10.1016/j.sbi.2003.08.002
Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW (2006) Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281:17789–17800. https://doi.org/10.1074/jbc.M600544200
Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ (1994) A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 265:1093–1095. https://doi.org/10.1126/science.8066447
Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR (2005) Alzheimer’s disease beta-secretase BACE1 is not a neuron-specific enzyme. J Neurochem 92:226–234. https://doi.org/10.1111/j.1471-4159.2004.02857.x
Burch ML, Getachew R, Osman N, Febbraio MA, Little PJ (2013) Thrombin-mediated proteoglycan synthesis utilizes both protein-tyrosine kinase and serine/threonine kinase receptor transactivation in vascular smooth muscle cells. J Biol Chem 288:7410–7419. https://doi.org/10.1074/jbc.M112.400259
Nikitovic D, Berdiaki A, Banos A, Tsatsakis A, Karamanos NK, Tzanakakis GN (2013) Could growth factor-mediated extracellular matrix deposition and degradation offer the ground for directed pharmacological targeting in fibrosarcoma? Curr Med Chem 20:2868–2880. https://doi.org/10.2174/0929867311320230003
Karkkainen I, Rybnikova E, Pelto-Huikko M, Huovila AP (2000) Metalloprotease-disintegrin (ADAM) genes are widely and differentially expressed in the adult CNS. Mol Cell Neurosci 15:547–560. https://doi.org/10.1006/mcne.2000.0848
Luo J (2005) The role of matrix metalloproteinases in the morphogenesis of the cerebellar cortex. Cerebellum 4:239–245. https://doi.org/10.1080/14734220500247646
Beroun A, Mitra S, Michaluk P, Pijet B, Stefaniuk M, Kaczmarek L (2019) MMPs in learning and memory and neuropsychiatric disorders. Cell Mol Life Sci 76:3207–3228. https://doi.org/10.1007/s00018-019-03180-8
Kärkkäinen I, Rybnikova E, Pelto-Huikko M, Huovila AP (2000) Metalloprotease-disintegrin (ADAM) genes are widely and differentially expressed in the adult CNS. Mol Cell Neurosci 15:547–560. https://doi.org/10.1006/mcne.2000.0848
Foscarin S, Raha-Chowdhury R, Fawcett JW, Kwok JCF (2017) Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging 9:1607–1622. https://doi.org/10.18632/aging.101256
Tewari BP, Sontheimer H (2019) Protocol to quantitatively assess the structural integrity of Perineuronal Nets ex vivo. Bio Protoc 9:e3234. https://doi.org/10.21769/BioProtoc.3234
Quirion R, Araujo D, Nair NP, Chabot JG (1988) Visualization of growth factor receptor sites in rat forebrain. Synapse 2:212–218. https://doi.org/10.1002/syn.890020307
Wamsley B, Fishell G (2017) Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci 18:299–309. https://doi.org/10.1038/nrn.2017.30
Lim L, Mi D, Llorca A, Marín O (2018) Development and functional diversification of Cortical Interneurons. Neuron 100:294–313. https://doi.org/10.1016/j.neuron.2018.10.009
Siebert JR, Conta Steencken A, Osterhout DJ (2014) Chondroitin sulfate proteoglycans in the nervous system: inhibitors to repair. Biomed Res Int 2014:845323. https://doi.org/10.1155/2014/845323
Takeuchi K, Yoshioka N, Higa Onaga S, Watanabe Y, Miyata S, Wada Y, Kudo C, Okada M, Ohko K, Oda K, Sato T, Yokoyama M, Matsushita N, Nakamura M, Okano H, Sakimura K, Kawano H, Kitagawa H, Igarashi M (2013) Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun 4:2740. https://doi.org/10.1038/ncomms3740
Butterfield KC, Conovaloff A, Caplan M, Panitch A (2010) Chondroitin sulfate-binding peptides block chondroitin 6-sulfate inhibition of cortical neurite growth. Neurosci Lett 478:82–87. https://doi.org/10.1016/j.neulet.2010.04.070
Takei N, Yokomaku D, Yamada T, Nagano T, Kakita A, Namba H, Ushiki T, Takahashi H, Nawa H (2022) EGF downregulates presynaptic maturation and suppresses synapse formation in Vitro and in vivo. Neurochem Res 47:2632–2644. https://doi.org/10.1007/s11064-021-03524-6
Nagano T, Namba H, Abe Y, Aoki H, Takei N, Nawa H (2007) In vivo administration of epidermal growth factor and its homologue attenuates developmental maturation of functional excitatory synapses in cortical GABAergic neurons. Eur J Neurosci 25:380–390. https://doi.org/10.1111/j.1460-9568.2007.05297.x
Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M (2017) Removal of Perineuronal nets unlocks juvenile plasticity through Network mechanisms of decreased inhibition and increased Gamma Activity. J Neurosci 37:1269–1283. https://doi.org/10.1523/jneurosci.2504-16.2016
Xu X, Roby KD, Callaway EM (2010) Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol 518:389–404. https://doi.org/10.1002/cne.22229
Gonchar Y, Burkhalter A (1997) Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7:347–358. https://doi.org/10.1093/cercor/7.4.347
Yamaguchi T, Ozawa H, Yamaguchi S, Hamaguchi S, Ueda S (2021) Calbindin-positive neurons co-express functional markers in a location-dependent Manner within the A11 region of the rat brain. Neurochem Res 46:853–865. https://doi.org/10.1007/s11064-020-03217-6
McDonald AJ, Hamilton PG, Barnstable CJ (2018) Perineuronal nets labeled by monoclonal antibody VC1.1 ensheath interneurons expressing parvalbumin and calbindin in the rat amygdala. Brain Struct Funct 223:1133–1148. https://doi.org/10.1007/s00429-017-1542-8
Horii-Hayashi N, Sasagawa T, Hashimoto T, Kaneko T, Takeuchi K, Nishi M (2015) A newly identified mouse hypothalamic area having bidirectional neural connections with the lateral septum: the perifornical area of the anterior hypothalamus rich in chondroitin sulfate proteoglycans. Eur J Neurosci 42:2322–2334. https://doi.org/10.1111/ejn.13024
Nawa H, Takei N (2006) Recent progress in animal modeling of immune inflammatory processes in schizophrenia: implication of specific cytokines. Neurosci Res 56:2–13. https://doi.org/10.1016/j.neures.2006.06.002
Nakamura JP, Schroeder A, Gibbons A, Sundram S, Hill RA (2022) Timing of maternal immune activation and sex influence schizophrenia-relevant cognitive constructs and neuregulin and GABAergic pathways. Brain Behav Immun 100:70–82. https://doi.org/10.1016/j.bbi.2021.11.006
Ketharanathan T, Pereira A, Reets U, Walker D, Sundram S (2021) Brain changes in NF-κB1 and epidermal growth factor system markers at peri-pubescence in the spiny mouse following maternal immune activation. Psychiatry Res 295:113564. https://doi.org/10.1016/j.psychres.2020.113564
Narihara I, Kitajo K, Namba H, Sotoyama H, Inaba H, Watanabe D, Nawa H (2021) Rat call-evoked electrocorticographic responses and Intercortical Phase Synchrony Impaired in a Cytokine-Induced Animal Model for Schizophrenia. Neurosci Res Doi. https://doi.org/10.1016/j.neures.2021.10.007
Jaaro-Peled H, Sawa A (2020) Neurodevelopmental factors in Schizophrenia. Psychiatr Clin North Am 43:263–274. https://doi.org/10.1016/j.psc.2020.02.010
Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT (2013) A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501:543–546. https://doi.org/10.1038/nature12485
Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK (2010) Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 30:14964–14971. https://doi.org/10.1523/jneurosci.4812-10.2010
Yoshioka N, Miyata S, Tamada A, Watanabe Y, Kawasaki A, Kitagawa H, Takao K, Miyakawa T, Takeuchi K, Igarashi M (2017) Abnormalities in perineuronal nets and behavior in mice lacking CSGalNAcT1, a key enzyme in chondroitin sulfate synthesis. Mol Brain 10:47. https://doi.org/10.1186/s13041-017-0328-5
Perry TL, Kish SJ, Buchanan J, Hansen S (1979) Gamma-aminobutyric-acid deficiency in brain of schizophrenic patients. Lancet 1:237–239. https://doi.org/10.1016/s0140-6736(79)90767-0
Bird ED, Spokes EG, Barnes J, MacKay AV, Iversen LL, Shepherd M (1977) Increased brain dopamine and reduced glutamic acid decarboxylase and choline acetyl transferase activity in schizophrenia and related psychoses. Lancet 2:1157–1158. https://doi.org/10.1016/s0140-6736(77)91542-2
Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, Deep-Soboslay A, Ye T, Li C, Kleinman JE, Wang KH, Lipska BK (2015) CHRNA7 and CHRFAM7A mRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. Am J Psychiatry 172:1122–1130. https://doi.org/10.1176/appi.ajp.2015.14080978
Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA (2016) Reduced labeling of parvalbumin neurons and Perineuronal Nets in the Dorsolateral Prefrontal cortex of subjects with Schizophrenia. Neuropsychopharmacology 41:2206–2214. https://doi.org/10.1038/npp.2016.24
Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo TU (2013) Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry 74:427–435. https://doi.org/10.1016/j.biopsych.2013.05.007
Pantazopoulos H, Woo TU, Lim MP, Lange N, Berretta S (2010) Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67:155–166. https://doi.org/10.1001/archgenpsychiatry.2009.196
Steullet P, Cabungcal JH, Bukhari SA, Ardelt MI, Pantazopoulos H, Hamati F, Salt TE, Cuenod M, Do KQ, Berretta S (2018) The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress. Mol Psychiatry 23:2057–2065. https://doi.org/10.1038/mp.2017.230
Gomes FV, Zhu X, Grace AA (2019) Stress during critical periods of development and risk for schizophrenia. Schizophr Res 213:107–113. https://doi.org/10.1016/j.schres.2019.01.030
Fujihara K (2023) Beyond the γ-aminobutyric acid hypothesis of schizophrenia. Front Cell Neurosci 17:1161608. https://doi.org/10.3389/fncel.2023.1161608
Ferguson BR, Gao WJ (2018) PV interneurons: critical regulators of E/I balance for Prefrontal Cortex-Dependent Behavior and Psychiatric disorders. Front Neural Circuits 12:37. https://doi.org/10.3389/fncir.2018.00037
Caballero A, Tseng KY (2016) GABAergic function as a limiting factor for Prefrontal Maturation during Adolescence. Trends Neurosci 39:441–448. https://doi.org/10.1016/j.tins.2016.04.010
Acknowledgements
We thank Ms. Eiko Kitayama and Dr. Meiko Kawamura for their technical assistance with the experiments.
Funding
This study was supported by KAKENHI under Grant Number 18K06460.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Y. I., H. N, and N. T. contributed to the study conception and design. Y.I. and Y.K. performed experiments. Materials were prepared or collected by Y. I., Y. K., and H. N. The first draft of the manuscript was written by Y. I. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional Review Board Statement
The animal study protocol was approved by the Animal Use and Care Committee of Niigata University (protocol code SA00246, 2018 May 7, SA00664, 2021 April 05, SA00835, 2023 January 30.)
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwakura, Y., Kobayashi, Y., Namba, H. et al. Epidermal Growth Factor Suppresses the Development of GABAergic Neurons Via the Modulation of Perineuronal Net Formation in the Neocortex of Developing Rodent Brains. Neurochem Res 49, 1347–1358 (2024). https://doi.org/10.1007/s11064-024-04122-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-024-04122-y