Abstract
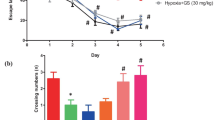
Abundant investigations have shown that hypobaric hypoxia (HH) causes cognitive impairment, mostly attributed to oxidative stress, inflammation, and apoptosis. HPN (4′-hydroxyl-2-subsitiuted phenylnitronyl nitroxide) is an excellent free radical scavenger with anti-inflammatory and anti-apoptotic activities. Our previous study has found that HPN exhibited neuroprotective effect on HH induced brain injury. In the present study, we examined the protective effect and potential mechanism of HPN on HH-induced cognitive impairment. Male mice were exposed to HH at 8000 m for 3 days with and without HPN treatment. Cognitive performance was assessed by the eight-arm radical maze. The histological changes were assayed by Nissle staining. The hippocampus cell apoptosis was detected by Tunnel staining. The levels of inflammatory cytokines and oxidative stress markers were detected. The expression of oxidative stress, inflammation-related and apoptosis-related proteins was determined by western blot. HPN administration significantly and mitigated HH induced histological damages and spatial memory loss with the evidence of decreased working memory error (WME), reference memory error (RME), total errors (TE) and total time (TT). In addition, HPN treatment significantly decreased the content of H2O2 and MDA, increased the levels of SOD, CAT, GSH-Px and GSH, and inhibited the synthesis of TNF-α, IL-1β and IL-6. Moreover, HPN administration could down-regulate the expression of NF-κB, TNF-α, Bax, and cleaved caspase-3 and up-regulate the expression of Nrf2, HO-1 and Bcl-2. The number of apoptotic cells was also significantly decreased in the hippocampus of mice in the HPN group. There results indicate that HPN improve HH-induced cognitive impairment by alleviating oxidative stress damage, suppressing inflammatory response and apoptosis and may be a powerful candidate compound for alleviating memory loss induced by HH.
Graphical Abstract











Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and supporting information.
References
Davis C, Hackett P (2017) Advances in the prevention and treatment of high altitude illness. Emerg Med Clin North Am 35:241–260. https://doi.org/10.1016/j.emc.2017.01.002
Erecińska M, Silver IA (2001) Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 128:263–276. https://doi.org/10.1016/s0034-5687(01)00306-1
de Aquino Lemos V, Antunes HK, dos Santos RV, Lira FS, Tufik S, de Mello MT (2012) High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 49:1298–1306. https://doi.org/10.1111/j.1469-8986.2012.01411.x
Kumari P, Kauser H, Wadhwa M, Roy K, Alam S, Sahu S, Kishore K, Ray K, Panjwani U (2018) Hypobaric hypoxia impairs cued and contextual fear memory in rats. Brain Res 1692:118–133. https://doi.org/10.1016/j.brainres.2018.04.026
Koester-Hegmann C, Bengoetxea H, Kosenkov D, Thiersch M, Haider T, Gassmann M, Schneider Gasser EM (2018) High-altitude cognitive impairment is prevented by enriched environment including exercise via VEGF signaling. Front Cell Neurosci 12:532. https://doi.org/10.3389/fncel.2018.00532
Ji W, Zhang Y, Luo J, Wan Y, Liu J, Ge RL (2021) Memantine ameliorates cognitive impairment induced by exposure to chronic hypoxia environment at high altitude by inhibiting excitotoxicity. Life Sci 270:119012. https://doi.org/10.1016/j.lfs.2020.119012
Chen C, Li B, Chen H, Qin Y, Cheng J, He B, Wan Y, Zhu D, Gao F (2022) Epigallocatechin-3-gallate ameliorated iron accumulation and apoptosis and promoted neuronal regeneration and memory/cognitive functions in the hippocampus induced by exposure to a chronic high-altitude hypoxia environment. Neurochem Res 47:2254–2262. https://doi.org/10.1007/s11064-022-03611-2
Hu SL, Xiong W, Dai ZQ, Zhao HL, Feng H (2016) Cognitive changes during prolonged stay at high altitude and its correlation with C-Reactive protein. PLoS ONE 11:e0146290. https://doi.org/10.1371/journal.pone.0146290
Kumar R, Jain V, Kushwah N, Dheer A, Mishra KP, Prasad D, Singh SB (2018) Role of DNA methylation in hypobaric hypoxia-induced neurodegeneration and spatial memory impairment. Ann Neurosci 25:191–200. https://doi.org/10.1159/000490368
Jain K, Prasad D, Singh SB, Kohli E (2015) Hypobaric hypoxia imbalances mitochondrial dynamics in rat brain hippocampus. Neurol Res Int 2015:742059. https://doi.org/10.1155/2015/742059
Muthuraju S, Maiti P, Solanki P, Sharma AK, Amitabh, Singh SB, Prasad D, Ilavazhagan G (2009) Acetylcholinesterase inhibitors enhance cognitive functions in rats following hypobaric hypoxia. Behav Brain Res 203:1–14. https://doi.org/10.1016/j.bbr.2009.03.026
Titus AD, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, Chattarji S, Raju TR (2007) Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145:265–278. https://doi.org/10.1016/j.neuroscience.2006.11.037
Baitharu I, Deep SN, Jain V, Barhwal K, Malhotra AS, Hota SK, Prasad D, Ilavazhagan G (2012) Corticosterone synthesis inhibitor metyrapone ameliorates chronic hypobaric hypoxia induced memory impairment in rat. Behav Brain Res 228:53–65. https://doi.org/10.1016/j.bbr.2011.11.030
Pena E, El Alam S, Siques P, Brito J (2022) Oxidative stress and diseases associated with high-altitude exposure. Antioxid (Basel) 11:267. https://doi.org/10.3390/antiox11020267
Chauhan G, Roy K, Kumar G, Kumari P, Alam S, Kishore K, Panjwani U, Ray K (2019) Distinct influence of COX-1 and COX-2 on neuroinflammatory response and associated cognitive deficits during high altitude hypoxia. Neuropharmacology 146:138–148. https://doi.org/10.1016/j.neuropharm.2018.11.026
Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G (2008) High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat 36:227–238. https://doi.org/10.1016/j.jchemneu.2008.07.003
Zhang X, Zhang X, Dang Z, Su S, Li Z, Lu D (2020) Cognitive protective mechanism of crocin pretreatment in rat submitted to acute high-altitude hypoxia exposure. Biomed Res Int 2020:3409679. https://doi.org/10.1155/2020/3409679
Zheng H, Su Y, Sun Y, Tang T, Zhang D, He X, Wang J (2019) Echinacoside alleviates hypobaric hypoxia-induced memory impairment in C57 mice. Phytother Res 33:1150–1160. https://doi.org/10.1002/ptr.6310
Jing L, Wu N, Zhang J, Da Q, Ma H (2022) Protective effect of 5,6,7,8-Tetrahydroxyflavone on high altitude cerebral edema in rats. Eur J Pharmacol 928:175121. https://doi.org/10.1016/j.ejphar.2022.175121
Bi W, Cai J, Xue P, Zhang Y, Liu S, Gao X, Li M, Wang Z, Baudy-Floc’h M, Green SA, Bi L (2008) Protective effect of nitronyl nitroxide-amino acid conjugates on liver ischemia-reperfusion induced injury in rats. Bioorg Med Chem Lett 18:1788–1794. https://doi.org/10.1016/j.bmcl.2008.02.030
Han WJ, Chen L, Wang HB, Liu XZ, Hu SJ, Sun XL, Luo C (2015) A novel nitronyl nitroxide with salicylic acid framework attenuates pain hypersensitivity and ectopic neuronal discharges in radicular low back pain. Neural plast. https://doi.org/10.1155/2015/752782
He SM, Lei YH, Wang JM, Geng LN, Wang SP, Zhao J, Hou YF (2020) The protective effect of nitronyl nitroxide radical on peroxidation of A549 cell damaged by iron overload. Mater sci Eng C Mater Biol Appl 108:110189. https://doi.org/10.1016/j.msec.2019.110189
Wang H, Gao P, Jing L, Qin X, Sun X (2012) The heart-protective mechanism of nitronyl nitroxide radicals on murine viral myocarditis induced by CVB3. Biochimie 94:1951–1959. https://doi.org/10.1016/j.biochi.2012.05.015
Guo J, Zhang Y, Zhang J, Liang J, Zeng L, Guo G (2012) Anticancer effect of tert-butyl-2(4,5-dihydrogen-4,4,5,5-tetramethyl-3-O-1H-imidazole-3-cationic-1-oxy l-2)-pyrrolidine-1-carboxylic ester on human hepatoma HepG2 cell line. Chemico-Biol Interact 199:38–48. https://doi.org/10.1016/j.cbi.2012.06.001
Wang H, Jia Y, Gao P, Cheng Y, Cheng M, Lu C, Zhou S, Sun X (2013) Synthesis, radioprotective activity and pharmacokinetics characteristic of a new stable nitronyl nitroxyl radical-NIT2011. Biochimie 95:1574–1581. https://doi.org/10.1016/j.biochi.2013.04.011
Wang H, Wang J, Yang Q, Zhang X, Gao P, Xu S, Sun X, Wang Y (2015) Synthesis of a novel nitronyl nitroxide radical and determination of its protective effects against infrasound-induced injury. Neurochem Res 40:1526–1536. https://doi.org/10.1007/s11064-015-1602-5
Shi T-y, Zhao D-q, Wang H-b, Feng S, Liu S-b, Xing J-h, Qu Y, Gao P, Sun X-l, Zhao M-g (2013) A new chiral pyrrolyl α-nitronyl nitroxide radical attenuates β-amyloid deposition and rescues memory deficits in a mouse model of Alzheimer disease. Neurotherapeutics 10:340–353. https://doi.org/10.1007/s13311-012-0168-z
Luo H, Sun W, Shao J, Ma H, Jia Z, Jing L (2020) Protective effect of nitronyl nitroxide against hypoxia-induced damage in PC12 cells Biochem cell biol 98:345–353. https://doi.org/10.1139/bcb-2019-0269
Fan PC, Ma HP, Jing LL, Li L, Jia ZP (2013) The antioxidative effect of a novel free radical scavenger 4’-hydroxyl-2-substituted phenylnitronyl nitroxide in acute high-altitude hypoxia mice. Biol Pharm Bull 36:917–924. https://doi.org/10.1248/bpb.b12-00854
Li M, Zhu Y, Li J, Chen L, Tao W, Li X, Qiu Y (2019) Effect and mechanism of verbascoside on hypoxic memory injury in plateau. Phytother Res 33:2692–2701. https://doi.org/10.1002/ptr.6443
Muthuraju S, Pati S (2014) Effect of hypobaric hypoxia on cognitive functions and potential therapeutic agents. Malays J Med Sci.: MJMS 21:41–45
Py G, Eydoux N, Lambert K, Chapot R, Koulmann N, Sanchez H, Bahi L, Peinnequin A, Mercier J, Bigard AX (2005) Role of hypoxia-induced anorexia and right ventricular hypertrophy on lactate transport and MCT expression in rat muscle. Metabolism 54:634–644. https://doi.org/10.1016/j.metabol.2004.12.007
Levin ED (1988) Psychopharmacological effects in the radial-arm maze. Neurosci Biobehav Rev 12:169–175. https://doi.org/10.1016/S0149-7634(88)80008-3
Chen H, Dong L, Chen X, Ding C, Hao M, Peng X, Zhang Y, Zhu H, Liu W (2022) Anti-aging effect of phlorizin on D-galactose-induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp Gerontol 163:111769. https://doi.org/10.1016/j.exger.2022.111769
Falla M, Papagno C, Dal Cappello T, Vögele A, Hüfner K, Kim J, Weiss EM, Weber B, Palma M, Mrakic-Sposta S, Brugger H, Strapazzon G (2021) A prospective evaluation of the acute effects of high altitude on cognitive and physiological functions in lowlanders. Front Physiol. https://doi.org/10.3389/fphys.2021.670278
Kushwah N, Jain V, Kadam M, Kumar R, Dheer A, Prasad D, Kumar B, Khan N (2021) Ginkgo biloba L. prevents hypobaric hypoxia-induced spatial memory deficit through small conductance calcium-activated potassium channel inhibition: the role of ERK/CaMKII/CREB signaling. Front Pharmacol 12:669701. https://doi.org/10.3389/fphar.2021.669701
Maiti P, Muthuraju S, Ilavazhagan G, Singh SB (2008) Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav Brain Res 189:233–243. https://doi.org/10.1016/j.bbr.2008.01.007
Tian JS, Zhai QJ, Zhao Y, Chen R, Zhao LD (2017) 2-(2-benzofuranyl)-2-imidazoline (2-BFI) improved the impairments in AD rat models by inhibiting oxidative stress, inflammation and apoptosis. J Integr Neurosci 16:385–400. https://doi.org/10.3233/jin-170032
Aimaier S, Tao Y, Lei F, Yupeng Z, Wenhui S, Aikemu A, Maimaitiyiming D (2023) Protective effects of the Terminalia bellirica tannin-induced Nrf2/HO-1 signaling pathway in rats with high-altitude pulmonary hypertension. BMC Complement Med Ther 23:150. https://doi.org/10.1186/s12906-023-03981-2
Xin X, Li Y, Liu H (2020) Hesperidin ameliorates hypobaric hypoxia-induced retinal impairment through activation of Nrf2/HO-1 pathway and inhibition of apoptosis. Sci Rep 10:19426. https://doi.org/10.1038/s41598-020-76156-5
Song TT, Bi YH, Gao YQ, Huang R, Hao K, Xu G, Tang JW, Ma ZQ, Kong FP, Coote JH, Chen XQ, Du JZ (2016) Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J Neuroinflamm 13:63. https://doi.org/10.1186/s12974-016-0528-4
Kammerer T, Faihs V, Hulde N, Stangl M, Brettner F, Rehm M, Horstmann M, Kröpfl J, Spengler C, Kreth S, Schäfer S (2020) Hypoxic-inflammatory responses under acute hypoxia: in Vitro experiments and prospective observational expedition trial. Int J Mol Sci. https://doi.org/10.3390/ijms21031034
Malacrida S, Giannella A, Ceolotto G, Reggiani C, Vezzoli A, Mrakic-Sposta S, Moretti S, Turner R, Falla M, Brugger H, Strapazzon G (2019) Transcription factors regulation in human peripheral white blood cells during hypobaric hypoxia exposure: an in-vivo experimental study. Sci Rep 9:9901. https://doi.org/10.1038/s41598-019-46391-6
Li D, Zhang L, Huang X, Liu L, He Y, Xu L, Zhang Y, Zhao T, Wu L, Zhao Y, Wu K, Wu Y, Fan M, Zhu L (2017) WIP1 phosphatase plays a critical neuroprotective role in Brain Injury Induced by High-Altitude hypoxic inflammation. Neurosci Bull 33:292–298. https://doi.org/10.1007/s12264-016-0095-9
Luan F, Li M, Han K, Ma Q, Wang J, Qiu Y, Yu L, He X, Liu D, Lv H (2019) Phenylethanoid glycosides of Phlomis younghusbandii Mukerjee ameliorate acute hypobaric hypoxia-induced brain impairment in rats. Mol Immunol 108:81–88. https://doi.org/10.1016/j.molimm.2019.02.002
Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G (2017) Neuroinflammation pathways: a general review. Int J Neurosci 127:624–633. https://doi.org/10.1080/00207454.2016.1212854
Bowie A, O’Neill LA (2000) Oxidative stress and nuclear factor-kappab activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 59:13–23. https://doi.org/10.1016/s0006-2952(99)00296-8
Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Patel NS, Caputi AP, Thiemermann C (2004) Tempol reduces the activation of nuclear factor-kappab in acute inflammation. Free Radic Res 38:813–819. https://doi.org/10.1080/10715760410001710829
Afjal MA, Abdi SH, Sharma S, Ahmad S, Fatima M, Dabeer S, Akhter J, Raisuddin S (2019) Anti-inflammatory role of tempol (4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl) in nephroprotection. Hum Exp Toxicol 38:713–723. https://doi.org/10.1177/0960327119836203
Ji W, Zhang Y, Ge RL, Wan Y, Liu J (2021) NMDA receptor-mediated excitotoxicity is involved in neuronal apoptosis and cognitive impairment induced by chronic hypobaric hypoxia exposure at high altitude. High Alt Med Biol 22:45–57. https://doi.org/10.1089/ham.2020.0127
Jing L, Shao J, Sun W, Lan T, Jia Z, Ma H, Wang H (2020) Protective effects of two novel nitronyl nitroxide radicals on heart failure induced by hypobaric hypoxia. Life Sci 248:116481. https://doi.org/10.1016/j.lfs.2019.05.037
Funding
This work was supported by the National Natural Science Foundation of China (81872796, 81202458, 81303097), Natural Science Foundation of Gansu Province (18JR3RA408) and Guizhou Province Science and Technology Plan Project (Qian ke He support [2020] 4Y128).
Author information
Authors and Affiliations
Contributions
LLJ and HBL: conceived and supervised the study; LLJ and HPM: designed experiments; QYD, JZ, SYZ and HPM: performed experiments; QYD and JZ: analyzed data; LLJ and QYD: wrote the manuscript; LLJ, HPM and HBL: revised manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
The current study was conducted strictly according to the principles and procedures approved by Animal Care and Use of the 940th Hospital of Joint Logistic Support Force of PLA (2018kyll015). All efforts were made to minimize the number of mice used and their suffering.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, L., Da, Q., Zhang, S. et al. Nitronyl Nitroxide Ameliorates Hypobaric Hypoxia-Induced Cognitive Impairment in Mice by Suppressing the Oxidative Stress, Inflammatory Response and Apoptosis. Neurochem Res 49, 785–799 (2024). https://doi.org/10.1007/s11064-023-04080-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04080-x