Abstract
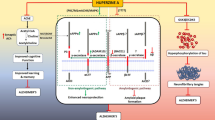
Xanthones are natural secondary metabolites that possess great potential as neuroprotective agents due to their prominent biological effects on Alzheimer’s disease (AD). However, their underlying mechanisms in AD remain unclear. This study aimed to systematically review the effects and mechanisms of xanthones in cell culture and animal studies, gaining a better understanding of their roles in AD. A comprehensive literature search was conducted in the Medline and Scopus databases using specific keywords to identify relevant articles published up to June 2023. After removing duplicates, all articles were imported into the Rayyan software. The article titles were screened based on predefined inclusion and exclusion criteria. Relevant full-text articles were assessed for biases using the OHAT tool. The results were presented in tables. Xanthones have shown various pharmacological effects towards AD from the 21 preclinical studies included. Cell culture studies demonstrated the anti-cholinesterase activity of xanthones, which protects against the loss of acetylcholine. Xanthones exhibited neuroprotective effects by promoting cell viability, reducing the accumulation of β-amyloid and tau aggregation. The administration of xanthones in animal models resulted in a reduction in neuronal inflammation by decreasing microglial and astrocyte burden. In terms of molecular mechanisms, xanthones prevented neuroinflammation through the modulation of signaling pathways, including TLR4/TAK1/NF-κB and MAPK pathways. Mechanisms such as activation of caspase-3 and -9 and suppression of endoplasmic reticulum stress were also reported. Despite the various neuroprotective effects associated with xanthones, there are limited studies reported on their underlying mechanisms in AD. Further studies are warranted to fully understand their potential roles in AD.

Similar content being viewed by others
Data Availability
The articles used for the current study are available from the corresponding author on reasonable request. The result of OHAT bias assessment is available in the supplementary information files.
Abbreviations
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- AMPK:
-
AMP-activated protein kinase
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid beta
- BACE1:
-
β-Site amyloid precursor protein cleaving enzyme 1
- Bax:
-
Bcl-2-associated X protein
- Bcl-2:
-
B-cell lymphoma 2
- BuChE:
-
Butyrylcholinesterase
- COX-2:
-
Cyclooxygenase-2
- DNA:
-
Deoxyribonucleic acid
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ERS:
-
Endoplasmic reticulum stress
- FDA:
-
Food and Drug Administration
- GSK:
-
Glycogen synthase kinase
- H2O2 :
-
Hydrogen peroxide
- HO:
-
Heme oxygenase
- IgG1:
-
Immunoglobulin G1
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- LPO:
-
Lipid peroxide
- LPS:
-
Lipopolysaccharide
- MeSH:
-
Medical Subject Headings
- mRNA:
-
Messenger ribonucleic acid
- NF-κB:
-
Nuclear factor kappa light chain enhancer of activated B cells
- NLRP3:
-
NLR family pyrin domain containing 3
- NO:
-
Nitric oxide
- OHAT:
-
Office of Health Assessment and Translation
- PGC-1 α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PICO:
-
Population, Intervention, Comparison and Outcome
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PRISMA-SR:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Systematic Reviews
- ROS:
-
Reactive oxygen species
- SIRT1:
-
Sirtuin 1
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor alpha
- TLR4:
-
Toll-like receptor-4
References
Guerchet M, Prince M, Prina M (2020) Numbers of people with dementia worldwide: an update to the estimates in the World Alzheimer Report 2015. https://www.alzint.org/u/numbers-people-with-dementia-2017.pdf. Accessed 30 Apr 2023
Adlard PA, Bush AI (2018) Metals and Alzheimer’s disease: how far have we come in the clinic? J Alzheimers Dis 62:1369–1379
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M (2019) Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomed 14:5541–5554
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14:101–115
Chen ZR, Huang JB, Yang SL, Hong FF (2022) Role of cholinergic signaling in Alzheimer’s disease. Molecules 27:1816
Kim DH, Brown RT, Ding EL, Kiel DP, Berry SD (2011) Dementia medications and risk of falls, syncope, and related adverse events: meta-analysis of randomized controlled trials. J Am Geriatr Soc 59:1019–1031
Cummings J, Lee G, Nahed P, Kambar M, Zhong K, Fonseca J, Taghva K (2022) Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement 8:e12295
Söderberg L, Johannesson M, Nygren P, Laudon H, Eriksson F, Osswald G, Möller C, Lannfelt L (2023) Lecanemab, aducanumab, and gantenerumab—binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer’s disease. Neurotherapeutics 20:195–206
Cummings J, Aisen P, Lemere C, Atri A, Sabbagh M, Salloway S (2021) Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res Ther 13:98
van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T (2023) Lecanemab in early Alzheimer’s disease. New Engl J Med 388:9–21
Shagufta AI (2016) Recent insight into the biological activities of synthetic xanthone derivatives. Eur J Med Chem 116:267–280
Pinto MM, Sousa ME, Nascimento MS (2005) Xanthone derivatives: new insights in biological activities. Curr Med Chem 12:2517–2538
Wang Y, Xia Z, Xu JR, Wang YX, Hou LN, Qiu Y, Chen HZ (2012) Α-mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 62:871–881
Khaw KY, Choi SB, Tan SC, Wahab HA, Chan KL, Murugaiyah V (2014) Prenylated xanthones from mangosteen as promising cholinesterase inhibitors and their molecular docking studies. Phytomedicine 21:1303–1309
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S (2014) PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res 14:579
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
National Institutes of Environmental Health Sciences (2019) Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/handbook/index.html. Accessed 30 Apr 2023
Jung K, Lee B, Han SJ, Ryu JH, Kim DH (2009) Mangiferin ameliorates scopolamine-induced learning deficits in mice. Biol Pharm Bull 32:242–246
Biradar SM, Joshi H, Chheda TK (2012) Neuropharmacological effect of mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer’s disease. Eur J Pharmacol 683:140–147
Du Z, Fanshi F, Lai YH, Chen JR, Hao E, Deng J, Hsiao CD (2019) Mechanism of anti-dementia effects of mangiferin in a senescence accelerated mouse (SAMP8) model. Biosci Rep 39:BSR20190488
Lei LY, Wang RC, Pan YL, Yue ZG, Zhou R, Xie P, Tang ZS (2021) Mangiferin inhibited neuroinflammation through regulating microglial polarization and suppressing NF-κB, NLRP3 pathway. Chin J Nat Med 19:112–119
Chen F, Wang N, Tian X, Su J, Qin Y, He R, He X (2023) The protective effect of mangiferin on formaldehyde-induced HT22 cell damage and cognitive impairment. Pharmaceutics 15:1568
Reyes-Fermín LM, González-Reyes S, Tarco-Álvarez NG, Hernández-Nava M, Orozco-Ibarra M, Pedraza-Chaverri J (2012) Neuroprotective effect of α-mangostin and curcumin against iodoacetate-induced cell death. Nutr Neurosci 15:34–41
Zhao LX, Wang Y, Liu T, Wang YX, Chen HZ, Xu JR, Qiu Y (2017) α-Mangostin decreases β-amyloid peptides production via modulation of amyloidogenic pathway. CNS Neurosci Ther 23:526–534
Guan H, Li J, Tan X, Luo S, Liu Y, Meng Y, Wu B, Zhou Y, Yang Y, Chen H, Hou L, Qiu Y, Li J (2020) Natural xanthone α-mangostin inhibits lps-induced microglial inflammatory responses and memory impairment by blocking the TAK1/NF-κB signaling pathway. Mol Nutr Food Res 64:e2000096
Tiang N, Ahad MA, Murugaiyah V, Hassan Z (2020) Xanthone-enriched fraction of Garcinia mangostana and α-mangostin improve the spatial learning and memory of chronic cerebral hypoperfusion rats. J Pharm Pharmacol 72:1629–1644
Ruankham W, Suwanjang W, Phopin K, Songtawee N, Prachayasittikul V, Prachayasittikul S (2022) Modulatory effects of alpha-mangostin mediated by SIRT1/3-FOXO3a pathway in oxidative stress-induced neuronal cells. Front Nutr 8:714463
Wang SN, Li Q, Jing MH, Alba E, Yang XH, Sabaté R, Han YF, Pi RB, Lan WJ, Yang XB, Chen JK (2016) Natural xanthones from Garcinia mangostana with multifunctional activities for the therapy of Alzheimer’s disease. Neurochem Res 41:1806–1817
Hu X, Liu C, Wang K, Zhao L, Qiu Y, Chen H, Hu J, Xu J (2022) Multifunctional anti-Alzheimer’s disease effects of natural xanthone derivatives: a primary structure-activity evaluation. Front Chem 10:842208
Alberdi E, Sánchez-Gómez MV, Ruiz A, Cavaliere F, Ortiz-Sanz C, Quintela-López T, Capetillo-Zarate E, Solé-Domènech S, Matute C (2018) Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxid Med Cell Longev 2018:2856063
Lee Y, Kim S, Oh Y, Kim Y-M, Chin Y-W, Cho J (2019) Inhibition of oxidative neurotoxicity and scopolamine-induced memory impairment by γ-mangostin: in vitro and in vivo evidence. Oxid Med Cell Longev 2019:3640753
Kong C, Jia L, Jia J (2022) γ-Mangostin attenuates amyloid-β42-induced neuroinflammation and oxidative stress in microglia-like BV2 cells via the mitogen-activated protein kinases signaling pathway. Eur J Pharmacol 917:174744
Xiong J, Liu XH, Bui VB, Hong ZL, Wang LJ, Zhao Y, Fan H, Yang GX, Hu JF (2014) Phenolic constituents from the leaves of Cratoxylum formosum ssp. pruniflorum. Fitoterapia 94:114–119
Gao XY, Wang SN, Yang XH, Lan WJ, Chen ZW, Chen JK, Xie JH, Han YF, Pi RB, Yang XB (2016) Gartanin protects neurons against glutamate-induced cell death in HT22 cells: independence of Nrf-2 but involvement of HO-1 and AMPK. Neurochem Res 41:2267–2277
Tonelli M, Catto M, Sabaté R, Francesconi V, Laurini E, Pricl S, Pisani L, Miniero DV, Liuzzi GM, Gatta E (2023) Thioxanthenone-based derivatives as multitarget therapeutic leads for Alzheimer’s disease. Eur J Med Chem 250:115169
Çalış İ, Becer E, Ünlü A, Aydın ZU, Hanoğlu A, Vatansever HS, Dönmez AA (2023) Comparative phytochemical studies on the roots of Polygala azizsancarii and P. peshmenii and neuroprotective activities of the two xanthones. Phytochemistry 210:113650
Abdallah HM, El Sayed NS, Sirwi A, Ibrahim SRM, Mohamed GA, Abdel Rasheed NO (2021) Mangostanaxanthone IV ameliorates streptozotocin-induced neuro-inflammation, amyloid deposition, and tau hyperphosphorylation via modulating PI3K/Akt/GSK-3β pathway. Biology 10:1298
Sethiya NK, Mishra SH (2014) Investigation of mangiferin, as a promising natural polyphenol xanthone on multiple targets of Alzheimer’s disease. J Biol Act Prod Nat 4:111–119
Negi JS, Bisht VK, Singh P, Rawat MSM, Joshi GP (2013) Naturally occurring xanthones: chemistry and biology. J Appl Chem 2013:621459
Chen Y, Bian Y, Wang J-W, Gong T-T, Ying Y-M, Ma L-F, Shan W-G, Xie X-Q, Zhan Z-J (2020) Effects of α-mangostin derivatives on the Alzheimer’s disease model of rats and their mechanism: a combination of experimental study and computational systems pharmacology analysis. ACS Omega 5:9846–9863
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795
Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287:C246–C256
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis 42(Suppl 3):S125–S152
Satoh T, McKercher SR, Lipton SA (2013) Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 65:645–657
Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A, Altucci L (2016) Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenet 8:1–21
Colonna M, Butovsky O (2017) Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468
Azam S, Jakaria M, Kim IS, Kim J, Haque ME, Choi DK (2019) Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol 10:1000
Bumrungpert A, Kalpravidh RW, Chuang CC, Overman A, Martinez K, Kennedy A, McIntosh M (2010) Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J Nutr 140:842–847
Miron J, Picard C, Frappier J, Dea D, Théroux L, Poirier J (2018) TLR4 gene expression and pro-inflammatory cytokines in Alzheimer’s disease and in response to hippocampal deafferentation in rodents. J Alzheimers Dis 63:1547–1556
Morrison DK (2012) MAP kinase pathways. Cold Spring Harb Perspect Biol 4:a011254
Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, Radhika S, Amit A, Venkateshwarlu K, Deepak M (2007) Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol 109:359–363
Chen WN, Yeong KY (2020) Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease. CNS Neurol Disord Drug Targets 19:85–93
Hampel H, Vassar R, De Strooper B, Hardy J, Willem M, Singh N, Zhou J, Yan R, Vanmechelen E, De Vos A, Nisticò R, Corbo M, Imbimbo BP, Streffer J, Voytyuk I, Timmers M, Tahami Monfared AA, Irizarry M, Albala B, Koyama A, Watanabe N, Kimura T, Yarenis L, Lista S, Kramer L, Vergallo A (2021) The β-secretase BACE1 in Alzheimer’s disease. Biol Psychiatry 89:745–756
Zhu K, Xiang X, Filser S, Marinković P, Dorostkar MM, Crux S, Neumann U, Shimshek DR, Rammes G, Haass C, Lichtenthaler SF, Gunnersen JM, Herms J (2018) Beta-site amyloid precursor protein cleaving enzyme 1 inhibition impairs synaptic plasticity via seizure protein 6. Biol Psychiatry 83:428–437
Bloom GS (2014) Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71:505–508
Qin J, Lan W, Liu Z, Huang J, Tang H, Wang H (2013) Synthesis and biological evaluation of 1,3-dihydroxyxanthone mannich base derivatives as anticholinesterase agents. Chem Cent J 7:78
Zhang Z, Guo J, Cheng M, Zhou W, Wan Y, Wang R, Fang Y, Jin Y, Liu J, Xie SS (2021) Design, synthesis, and biological evaluation of novel xanthone-alkylbenzylamine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem 213:113154
Funding
This manuscript received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualisation: YHY, SH, MSH; writing-original draft preparation: PLW, YHY; writing: PLW, SH, SLJT, MSH, YHY; review and editing: YHY, SLJT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pang, L.W., Hamzah, S., Tan, S.L.J. et al. The Effects and Mechanisms of Xanthones in Alzheimer’s Disease: A Systematic Review. Neurochem Res 48, 3485–3511 (2023). https://doi.org/10.1007/s11064-023-04005-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04005-8