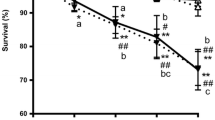
Abstract
Progressive neurodegenerative disorders such as Parkinson Disease (PD) lack curative or long-term treatments. At the same time, the increase of the worldwide elderly population and, consequently, the extension in the prevalence of age-related diseases have promoted research interest in neurodegenerative disorders. Caenorhabditis elegans is a free-living nematode widely used as an animal model in studies of human diseases. Here we evaluated cannabidiol (CBD) as a possible neuroprotective compound in PD using the C. elegans models exposed to reserpine. Our results demonstrated that CBD reversed the reserpine-induced locomotor alterations and this response was independent of the NPR-19 receptors, an orthologous receptor for central cannabinoid receptor type 1. Morphological alterations of cephalic sensilla (CEP) dopaminergic neurons indicated that CBD also protects neurons from reserpine-induced degeneration. That is, CBD attenuates the reserpine-induced increase of worms with shrunken soma and dendrites loss, increasing the number of worms with intact CEP neurons. Finally, we found that CBD also reduced ROS formation and α-syn protein accumulation in mutant worms. Our findings collectively provide new evidence that CBD acts as neuroprotector in dopaminergic neurons, reducing neurotoxicity and α-syn accumulation highlighting its potential in the treatment of PD.










Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG (1982) Action of cannabidiol on the anxiety and other effects produced by ∆9-THC in normal subjects. Psychopharmacology 76:245–250. https://doi.org/10.1007/BF00432554
Carlini EA, Cunha JM (1981) Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol 21:417–427. https://doi.org/10.1002/j.1552-4604.1981.tb02622.x
Rajesh M, Mukhopadhyay P, Bátkai S et al (2010) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56:2115–2125. https://doi.org/10.1016/j.jacc.2010.07.033
Peres FF, Lima AC, Hallak JE et al (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9:482. https://doi.org/10.3389/fphar.2018.00482
Barata L, Arruza L, Rodríguez MJ, Aleo E, Vierge E et al (2019) Neuroprotection by cannabidiol and hypothermia in a piglet model of newborn hypoxic-ischemic brain damage. Neuropharmacology 146:1–11. https://doi.org/10.1016/j.neuropharm.2018.11.020
Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J (2005) Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 19:96–107. https://doi.org/10.1016/j.nbd.2004.11.009
Chagas MHN, Eckeli AL, Zuardi AW et al (2014) Cannabidiol can improve complex sleep-related behaviors associated with rapid eye movement sleep behavior disorder in parkinson’s disease patients: a case series. J Clin Pharm Ther 39:564–566. https://doi.org/10.1111/jcpt.12179
Dugger BN, Dickson DW (2017) Pathology of neurodegenerative Diseases. Cold Spring Harb Perspect Biol 9:a028035. https://doi.org/10.1101/cshperspect.a028035
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL et al (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15:565–581. https://doi.org/10.1038/s41582-019-0244-7
Pupyshev AB, Korolenko TA, Akopyan AA, Amstislavskaya TG, Tikhonova MA (2018) Suppression of autophagy in the brain of transgenic mice with overexpression of А53Т-mutant α-synuclein as an early event at synucleinopathy progression. Neurosci Lett 672:140–144. https://doi.org/10.1016/j.neulet.2017.12.001
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047. https://doi.org/10.1126/science.276.5321.204
Braak H, Braak E, Yilmazer D, Schultz C, De Vos RA, Jansen EN (1995) Nigral and extranigral pathology in Parkinson’s disease. J Neural Transm 46:15–31
Dirkx MF, den Ouden HE, Aarts E, Timmer MH, Bloem BR, Toni I, Helmich RC (2017) Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain aww331. https://doi.org/10.1093/brain/aww331
Kalyn M, Hua K, Mohd Noor S, Wong CED, Ekker M (2019) Comprehensive analysis of neurotoxin-induced ablation of dopaminergic neurons in zebrafish larvae. Biomedicines 8:1. https://doi.org/10.3390/biomedicines8010001
Lei H, Ren R, Sun Y, Zhang K, Zhao X, Ablat N, Pu X (2020) Neuroprotective effects of safflower flavonoid extract in 6-hydroxydopamine-induced model of Parkinson’s Disease may be related to its anti-inflammatory action. Molecules 25:5206. https://doi.org/10.3390/molecules25215206
Abílio VC, Silva RH, Carvalho RC, Grassl C et al (2004) Important role of striatal catalase in aging and reserpine-induced oral dyskinesia. Neuropharmacology 47:263–272. https://doi.org/10.1016/j.neuropharm.2004.04.003
Reckziegel P, Peroza LR, Schaffer LF et al (2013) Gallic acid decreases vacuous chewing movements induced by reserpine in rats. Pharmacol Biochem Behav 104:132–137. https://doi.org/10.1016/j.pbb.2013.01.001
Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS (1995) Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J Neurosci 15:3429–3439. https://doi.org/10.1523/JNEUROSCI.15-05-03429.1995
Wang S, Duan M, Guan K, Zhou X, Zheng M, Shi X et al (2019) Developmental neurotoxicity of reserpine exposure in zebrafish larvae (Danio rerio). Comp Biochem Physiol C 223:115–123. https://doi.org/10.1016/j.cbpc.2019.05.008
Reckziegel P, Chen P, Caito S, Gubert P, Soares FA, Fachinetto R, Aschner M (2016) Extracellular dopamine and alterations on dopamine transporter are related to reserpine toxicity in Caenorhabditis elegans. Arch Toxicol 90:633–645. https://doi.org/10.1007/s00204-015-1451-7
Vijayan B, Raj V, Nandakumar S, Kishore A, Thekkuveettil A (2019) Spermine protects alpha-synuclein expressing dopaminergic neurons from manganese-induced degeneration. Cell Biol Toxicol 35:147–159. https://doi.org/10.1007/s10565-018-09449-1
Li J, Le W (2013) Modeling neurodegenerative diseases in Caenorhabditis elegans. Exp Neurol 250:94–103. https://doi.org/10.1016/j.expneurol.2013.09.024
Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163:215–226. https://doi.org/10.1002/cne.901630207
Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W (2000) Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res 10:703–713. https://doi.org/10.1101/gr.10.5.703
McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD (2007) Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci 27:14216–14227. https://doi.org/10.1523/JNEUROSCI.2992-07.2007
C. elegans Sequencing Consortium (1998) Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282:2012–8. https://doi.org/10.1126/science.282.5396.2012
Oakes MD, Law WJ, Clark T, Bamber BA, Komuniecki R (2017) Cannabinoids activate monoaminergic signaling to modulate key C. elegans behaviors. J Neurosci 37:2859–2869. https://doi.org/10.1523/JNEUROSCI.3151-16.2017
Peres FF, Levin R, Suiama MA, Diana MC, Gouvêa DA et al (2016) Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front Pharmacol 7:343. https://doi.org/10.3389/fphar.2016.00343
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94. https://doi.org/10.1093/genetics/77.1.71
Wang MC, Rourke EJ, Ruvkun G (2008) Fat metabolism links germline stem cells and longevity in C. elegans. Science 322:957–960. https://doi.org/10.1126/science.1162011
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26:619–631. https://doi.org/10.1016/s0896-6273(00)81199-x
Liao VHC et al (2011) Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev 132:480–487. https://doi.org/10.1016/j.mad.2011.07.008
Ortiz-Padilla S, Soto-Orduño E, Barrios ME et al (2020) Blockade of the dopaminergic neurotransmission with AMPT and reserpine induces a differential expression of genes of the dopaminergic phenotype in substantia nigra. Neuropharmacology 166:107920. https://doi.org/10.1016/j.neuropharm.2019.107920
Manzanza NO, Sedlackova L, Kalaria RN (2021) Alpha-synuclein post-translational modifications: implications for pathogenesis of lewy body disorders. Front Aging Neurosci 13:690293. https://doi.org/10.3389/fnagi.2021.690293
Komuniecki RW, Hobson RJ, Rex EB, Hapiak VM, Komuniecki PR (2004) Biogenic amine receptors in parasitic nematodes: what can be learned from Caenorhabditis elegans? Mol Biochem Parasitol 137:1–11. https://doi.org/10.1016/j.molbiopara.2004.05.010
Maalouf M, Rho JM, Mattson MP (2009) The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 5:293–315. https://doi.org/10.1016/j.brainresrev.2008.09.002
Pani G (2015) Neuroprotective effects of dietary restriction: evidence and mechanisms. Semin Cell Dev Biol 40:106–114. https://doi.org/10.1016/j.semcdb.2015.03.004
Hansen M et al (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4:2. https://doi.org/10.1371/journal.pgen.0040024
Zhang S, Li F, Zhou T, Wang G, Li Z (2020) Caenorhabditis elegans as a useful model for studying aging mutations. Front Endocrinol 11:554994. https://doi.org/10.3389/fendo.2020.554994
Raizen DM, Lee RY, Avery L (1995) Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141:1365–1382. https://doi.org/10.1093/genetics/141.4.1365
Lakowski B, Hekimi S (1998) The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A 95:13091–13096. https://doi.org/10.1073/pnas.95.22.13091
Dall KB, Færgeman NJ (2019) Metabolic regulation of lifespan from a C. elegans perspective. Genes Nutr 14:25. https://doi.org/10.1186/s12263-019-0650-x
Land MH et al (2021) Effect of cannabidiol on the long-term toxicity and lifespan in the preclinical model Caenorhabditis elegans. Cannabis Cannabinoid Res 6:522–527. https://doi.org/10.1089/can.2020.0103
Wang YA, van Sluijs L, Nie Y, Sterken MG, Harvey SC, Kammenga JE (2020) Genetic variation in Complex Traits in transgenic α-Synuclein strains of Caenorhabditis elegans. Genes (Basel) 11:778. https://doi.org/10.3390/genes11070778
Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB (1999) The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci 19:72–84. https://doi.org/10.1523/JNEUROSCI.19-01-00072.1999
Vanin AP, Tamagno WA, Alves C, Mesacasa L, Santin LF, Sutorillo NT, Bilibio D, Müller C, Galon L, Kaizer RR (2022) Neuroprotective potential of Cannabis sativa-based oils in Caenorhabditis elegans. Sci Rep 12:15376. https://doi.org/10.1038/s41598-022-19598-3
Bisogno T, Hanus L, de Petrocellis L et al (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134:845–852. https://doi.org/10.1038/sj.bjp.0704327
Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ (2015) Molecular targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 12:699–730. https://doi.org/10.1007/s13311-015-0377-3
Cheer JF, Heien ML, Garris PA, Carelli RM, Wightman RM (2005) Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: implications for intracranial self-stimulation. Proc Natl Acad Sci U S A 102:19150–19155. https://doi.org/10.1073/pnas.0509607102
Zygmunt PM, Ermund A, Movahed P, Andersson DA, Simonsen C et al (2013) Monoacylglycerols activate TRPV1—a link between phospholipase C and TRPV1. PLoS ONE 8:e81618. https://doi.org/10.1371/journal.pone.0081618
De Luca MA, Valentini V, Bimpisidis Z, Cacciapaglia F, Caboni P, Di Chiara G (2014) Endocannabinoid 2-arachidonoylglycerol self-administration by sprague-dawley rats and stimulation of in vivo dopamine transmission in the nucleus accumbens shell. Front Psychiatry 5:140. https://doi.org/10.3389/fpsyt.2014.00140
Wang H, Treadway T, Covey DP, Cheer JF, Lupica CR (2015) Cocaine induced endocannabinoid mobilization in the ventral tegmental area. Cell Rep 12:1997–2008. https://doi.org/10.1016/j.celrep.2015.08.041
Oakes M, Law WJ, Komuniecki R (2019) Cannabinoids stimulate the TRP channel-dependent release of both serotonin and dopamine to modulate behavior in C. elegans. J Neurosci 39:4142–4152. https://doi.org/10.1523/JNEUROSCI.2371-18.2019
Maor G, Dubreuil RR, Feany MB (2023) α-Synuclein promotes neuronal dysfunction and death by disrupting the binding of ankyrin to ß-spectrin. J Neurosci. https://doi.org/10.1523/JNEUROSCI.1922-22.2022
Scudamore O, Ciossek T (2018) Increased oxidative stress exacerbates α-synuclein aggregation in vivo. J Neuropathol Exp Neurol 77:443–453. https://doi.org/10.1093/jnen/nly024
Puspita L, Chung SY, Shim J (2017) Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain 10:1–12. https://doi.org/10.1186/s13041-017-0340-9
Ulusoy A et al (2012) Dysregulated dopamine storage increases the vulnerability to α-synuclein in nigral neurons. Neurobiol Dis 47:367–377. https://doi.org/10.1016/j.nbd.2012.05.012
Pohl F et al (2019) GST-4-dependent suppression of neurodegeneration in C. elegans models of Parkinson’s and Machado-Joseph disease by rapeseed pomace extract supplementation. Front NeuroSci 13:1091. https://doi.org/10.3389/fnins.2019.01091
Yakunin E et al (2014) The regulation of catalase activity by PPAR γ is affected by α-synuclein. Ann Clin Transl Neurol 1:145–159. https://doi.org/10.1002/acn3.38
O’sullivan SE et al (2009) Time-dependent vascular actions of cannabidiol in the rat aorta. Eur J Pharmacol 612:61–68. https://doi.org/10.1016/j.ejphar.2009.03.010
Patricio F et al (2020) Cannabidiol as a therapeutic target: evidence of its neuroprotective and neuromodulatory function in Parkinson’s disease. Front Pharmacol 11:595635. https://doi.org/10.3389/fphar.2020.595635
Acknowledgements
We thank Marina Yukari Kubota, Ingrid Kazue Mizuno Watanabe, Vanessa Gusmão, Cícero Ramos dos Santos, Maria de Lourdes Santos, Elizabeth Kanashiro for technical assistance.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP: 2017/10863-7; 2019/14722-4 (GJSP); 2019/02821-8 (SSS). Confocal microscope Zeiss LSM 780 and Leica Microsystems TCSSP8 facility from the Instituto de Farmacologia e Biologia Molecular (INFAR) was supported by Financiadora de Estudos e Projetos (FINEP) and FAPESP.Post-doctoral (AHFFL) and Doctoral (ECG) fellowships were supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Post-doctoral (AGE) fellowship was supported by FAPESP: 2020/08840-1.
Author information
Authors and Affiliations
Contributions
ECG, PR, GJSP Conception and design of all experimental protocols. ECG, AGE, AHFFL, CAC, VCA, AWZ, JECH, JAC, CB, SSS, PR, GJSP Development of methodology performed. ECG, AGE, AHFFL, CAC, PR, GJSP Data acquisition, analysis and interpretation of results related to worm behavior on locomotion, defecation, egg production and egg laying parameters, fluorescence microscopy and western blotting. ECG, AGE, AHFFL, CAC, VCA, AWZ, JECH, JAC, CB, SSS, PR, GJSP Writing and/or revision of the manuscript. Study supervision: PR, GJSP All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interest
JAC reported receiving grants from the National Institute of Translational Science and Technology in Medicine and personal fees from the National Council for Scientific and Technological Development (CNPq 1A) during the conduct of the study, being a co-owner of a patent for fluorinated cannabidiol compounds (licensed to Phytecs) and having a patent pending for a cannabinoid-containing oral pharmaceutical composition outside the submitted work. JAC is a consultant and/or has received speaker fees and/or sits on the advisory board and/or receives research funding from Janssen-Cilag, Torrent, GreenCare, PurMed Global, BioSynthesis Pharma Group (BSPG), and Prati-Donaduzzi. JAC reported receiving grants from FAPESP. The other authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Cruz Guedes, E., Erustes, A.G., Leão, A.H.F.F. et al. Cannabidiol Recovers Dopaminergic Neuronal Damage Induced by Reserpine or α-synuclein in Caenorhabditis elegans. Neurochem Res 48, 2390–2405 (2023). https://doi.org/10.1007/s11064-023-03905-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03905-z