Abstract
Values of binding potentials (BPND) of dopamine D2/3 receptors differ in different regions of the brain, but we do not know with certainty how much of this difference is due either to different receptor numbers, or to different affinities of tracers to the receptors, or to both. We tested the claim that both striatal and extrastriatal dopamine D2/3 receptor availabilities vary with age in vivo in humans by determining the values of BPND of the specific radioligand [11C]raclopride. We determined values of BPND in striatal and extrastriatal volumes-of-interest (VOI) with the same specific receptor radioligand. We estimated values of BPND in individual voxels of brains of healthy volunteers in vivo, and we obtained regional averages of VOI by dynamic positron emission tomography (PET). We calculated average values of BPND in caudate nucleus and putamen of striatum, and in frontal, occipital, parietal, and temporal cortices of the forebrain, by means of four methods, including the ERLiBiRD (Estimation of Reversible Ligand Binding and Receptor Density) method, the tissue reference methods of Logan and Logan-Ichise, respectively, and the SRTM (Simplified Reference Tissue Method). Voxelwise generation of parametric maps of values of BPND used the multi-linear regression version of SRTM. Age-dependent changes of the binding potential presented with an inverted U-shape with peak binding potentials reached between the ages of 20 and 30. The estimates of BPND declined significantly with age after the peak in both striatal and extrastriatal regions, as determined by all four methods, with the greatest decline observed in posterior (occipital and parietal) cortices (14% per decade) and the lowest decline in caudate nucleus (3% per decade). The sites of the greatest declines are of particular interest because of the clinical implications.




Similar content being viewed by others
Data Availability
The data sets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
References
Gründer G, Carlsson A, Wong DF (2003) Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry 60(10):974–977
Lidow MS, Williams GV, Goldman-Rakic PS (1998) The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci 19(4):136–140
Wong DF, Brašić JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y et al (2008) Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology 33(6):1239–1251
Wong DF, Gjedde A, Wagner HN Jr, Dannals RF, Douglass KH, Links JM et al (1986) Quantification of neuroreceptors in the living human brain. II. Inhibition studies of receptor density and affinity. J Cereb Blood Flow Metab 6(2):147–153
Wong DF, Wagner HN, Tune LE, Dannals RF, Pearlson GD, Links JM et al (1986) Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science 234(4783):1558–1563
Nakajima S, Caravaggio F, Boileau I, Chung JK, Plitman E, Gerretsen P et al (2015) Lack of age-dependent decrease in dopamine D3 receptor availability: a [11C]-(+)-PHNO and [11C]-raclopride positron emission tomography study. J Cereb Blood Flow Metab 35(11):1812–1818
Wong DF, Wagner HN, Dannals RF, Links JM, Frost JJ, Ravert HT et al (1984) Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science 226(4681):1393–1396
Nyberg L, Karalija N, Salami A, Andersson M, Wåhlin A, Kaboovand N et al (2016) Dopamine D2 receptor availability is linked to hippocampal–caudate functional connectivity and episodic memory. Proc Natl Acad Sci USA 113(28):7918–7923
Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T et al (2006) Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[β-11C] DOPA. Life Sci 79(8):730–736
Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT et al (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34(5):721–733
Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding Y-S et al (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155(3):344–349
Antonini A, Leenders KL, Reist H, Thomann R, Beer H-F, Locher J (1993) Effect of age on D2 dopamine receptors in normal human brain measured by positron emission tomography and 11C-raclopride. Arch Neurol 50(5):474–480
Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, MacGregor RR et al (1996) Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res: Neuroimaging 67(1):11–16
Wang G-J, Volkow ND, Fowler JS, Logan J, Gur R, Netusil N et al (1996) Age associated decrements in dopamine D2 receptors in thalamus and in temporal insula of human subjects. Life Sci 59(1):PL31–PL35
Wong DF, Pearlson GD, Tune LE, Young LT, Meltzer CC, Dannals RF et al (1997) Quantification of neuroreceptors in the living human brain: IV. Effect of aging and elevations of D2-like receptors in schizophrenia and bipolar illness. J Cereb Blood Flow Metab 17(3):331–342
Gjedde A, Wong D, Wagner H Jr (1986) Transient analysis of irreversible and reversible tracer binding in human brain in vivo. PET and NMR: New perspectives in neuroimaging and in clinical neurochemistry. AR Liss, New York, pp 223–235
Siessmeier T, Zhou Y, Buchholz H-G, Landvogt C, Vernaleken I, Piel M et al (2005) Parametric mapping of binding in human brain of D2 receptor ligands of different affinities. J Nucl Med 46(6):964–972
Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR (2017) Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging 57:36–46
Inoue M, Suhara T, Sudo Y, Okubo Y, Yasuno F, Kishimoto T et al (2001) Age-related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci 69(9):1079–1084
Kaasinen V, Kemppainen N, Någren K, Helenius H, Kurki T, Rinne JO (2002) Age-related loss of extrastriatal dopamine D2-like receptors in women. J Neurochem 81(5):1005–1010
Kaasinen V, Någren K, Hietala J, Oikonen V, Vilkman H, Farde L et al (2000) Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson’s disease. Neurology 54(7):1482–1487
Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H et al (2000) Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 21(5):683–688
Joyce JN, Myers AJ, Gurevich E (1998) Dopamine D2 receptor bands in normal human temporal cortex are absent in Alzheimer’s disease. Brain Res 784(1–2):7–17
Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ et al (2011) Microglial activation in regions related to cognitive function predicts disease onset in Huntington’s disease: a multimodal imaging study. Hum Brain Mapp 32(2):258–270
Ribeiro M-J, Thobois S, Lohmann E, Du Montcel ST, Lesage S, Pelissolo A et al (2009) A multitracer dopaminergic PET study of young-onset parkinsonian patients with and without parkin gene mutations. J Nucl Med 50(8):1244–1250
Seaman KL, Smith CT, Juarez EJ, Dang LC, Castrellon JJ, Burgess LL et al (2019) Differential regional decline in dopamine receptor availability across adulthood: linear and nonlinear effects of age. Hum Brain Mapp 40(10):3125–3138
Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP et al (1987) Human brain dopamine receptors in children and aging adults. Synapse 1(5):399–404
Gjedde A (2003) Modelling metabolite and tracer kinetics. In: Feinendegen LE, Shreeve WW, Eckelman WC, Bahk Y-W, Wagner HN (eds) Molecular nuclear medicine. Berlin, Springer, pp 121–169
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain : 3-dimensional proportional system : an approach to cerebral imagin. Stuttgart
Griffin JP, Posner J, Barker GR (2013) Textbook of pharmaceutical medicine, 7th edn. Wiley, Hoboken
Møller M, Jakobsen S, Gjedde A (2007) Parametric and regional maps of free serotonin 5HT 1A receptor sites in human brain as function of age in healthy humans. Neuropsychopharmacology 32(8):1707–1714
Rosa-Neto P, Gjedde A, Olsen AK, Jensen SB, Munk OL, Watanabe H et al (2004) MDMA-evoked changes in [11C] raclopride and [11C] NMSP binding in living pig brain. Synapse 53(4):222–233
Ichise M, Fujita M, Seibyl JP, Verhoeff NPL, Baldwin RM, Zoghbi SS et al (1999) Graphical analysis and simplified quantification of striatal and extrastriatal dopamine D2 receptor binding with [123I] epidepride SPECT. J Nucl Med 40(11):1902–1912
Logan J, Fowler JS, Volkow ND, Wang G-J, Ding Y-S, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16(5):834–840
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4(3):153–158
Winterdahl M, Noer O, Orlowski D, Schacht AC, Jakobsen S, Alstrup AKO, Gjedde A, Landau AM (2019) Sucrose intake lowers μ-opioid and dopamine D2/3 receptor availability in porcine brain. Sci Rep 9(1):16918. https://doi.org/10.1038/s41598-019-53430-9
Borghammer P, Jonsdottir KY, Cumming P, Ostergaard K, Vang K, Ashkanian M, Vafaee M, Iversen P, Gjedde A (2008) Normalization in PET group comparison studies—the importance of a valid reference region. Neuroimage 40(2):529–540. https://doi.org/10.1016/j.neuroimage.2007.12.057
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27(9):1533–1539
Zhou Y, Endres CJ, Brašić JR, Huang S-C, Wong DF (2003) Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage 18(4):975–989
Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996) A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4(1):58–73
Mozley PD, Kim H-J, Gur RC, Tatsch K, Muenz LR, McElgin WT et al (1996) Iodine-123-IPT SPECT imaging of CNS dopamine transporters: nonlinear effects of normal aging on striatal uptake values. J Nucl Med 37(12):1965–1970
Farde L, Eriksson L, Blomquist G, Halldin C (1989) Kinetic analysis of central [11C] raclopride binding to D2-dopamine receptors studied by PET—a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 9(5):696–708
Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K et al (1999) Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmacol 2(2):73–82
Phan JA, Wong DF, Chang NH, Kumakura Y, Nandi A, Bauer WR, Gjedde A (2022) Transient equilibrium determination of dopamine D2/D3 receptor densities and affinities in brain. Front Nucl Med. In press
Alakurtti K, Johansson JJ, Joutsa J, Laine M, Bäckman L, Nyberg L et al (2015) Long-term test–retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [11C] raclopride and high-resolution PET. J Cereb Blood Flow Metab 35(7):1199–1205
Laruelle M, Slifstein M, Huang Y (2003) Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imag Biol 5(6):363–375
Stokes PR, Egerton A, Watson B, Reid A, Breen G, Lingford-Hughes A et al (2010) Significant decreases in frontal and temporal [11C]-raclopride binding after THC challenge. Neuroimage 52(4):1521–1527
Rinne J (1987) Muscarinic and dopaminergic receptors in the aging human brain. Brain Res 404(1–2):162–168
Rinne JO, Hietala J, Ruotsalainen U, Säkö E, Laihinen A, Någren K et al (1993) Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C] raclopride. J Cereb Blood Flow Metab 13(2):310–314
Rinne JO, Lönnberg P, Marjamäki P (1990) Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res 508(2):349–352
Joyce J, Janowsky A, Neve K (1991) Characterization and distribution of [125I] epidepride binding to dopamine D2 receptors in basal ganglia and cortex of human brain. J Pharmacol Exp Ther 257(3):1253–1263
Farde L, Hall H, Pauli S, Halldin C (1995) Variability in D2-dopamine receptor density and affinity: a PET study with [11C] raclopride in man. Synapse 20(3):200–208
Kessler RM, Whetsell WO, Ansari MS, Votaw JR, de Paulis T, Clanton JA et al (1993) Identification of extrastriatal dopamine D2 receptors in post mortem human brain with [125I] epidepride. Brain Res 609(1–2):237–243
Bettinardi V, Castiglioni I, De Bernardi E, Gilardi M (2014) PET quantification: strategies for partial volume correction. Clinical and Translational Imaging 2(3):199–218
Rinne JO, Laihinen A, Ruottinen H, Ruotsalainen U, Någren K, Lehikoinen P et al (1995) Increased density of dopamine D2 receptors in the putamen, but not in the caudate nucleus in early Parkinson’s disease: a PET study with [11C] raclopride. J Neurol Sci 132(2):156–161
Buchsbaum MS, Christian BT, Lehrer DS, Narayanan TK, Shi B, Mantil J et al (2006) D2/D3 dopamine receptor binding with [F-18] fallypride in thalamus and cortex of patients with schizophrenia. Schizophr Res 85(1–3):232–244
Hirvonen J, van Erp TG, Huttunen J, Aalto S, Någren K, Huttunen M et al (2005) Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry 62(4):371–378
Fazio P, Schain M, Mrzljak L, Amini N, Nag S, Al-Tawil N et al (2017) Patterns of age related changes for phosphodiesterase type-10A in comparison with dopamine D2/3 receptors and sub-cortical volumes in the human basal ganglia: a PET study with 18F-MNI-659 and 11C-raclopride with correction for partial volume effect. Neuroimage 152:330–339
Rominger A, Cumming P, Xiong G, Koller G, Böning G, Wulff M, Zwergal A, Förster S, Reilhac A, Munk O, Soyka M, Wängler B, Bartenstein P, la Fougère C, Pogarell O (2012) [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict Biol 17(2):490–503. https://doi.org/10.1111/j.1369-1600.2011.00355.x
Kuwabara H, McCaul ME, Wand GS, Earley CJ, Allen RP, Weerts EM et al (2012) Dissociative changes in the Bmax and KD of dopamine D2/D3 receptors with aging observed in functional subdivisions of the striatum: a revisit with an improved data analysis method. J Nucl Med 53(5):805–812
Gjedde A, Kumakura Y, Cumming P, Linnet J, Møller A (2010) Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci USA 107(8):3870–3875
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS et al (2000) Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97(14):8104–8109
Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL et al (1997) Imaging D 2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17(3):162–167
Acknowledgements
We thank Per Borghammer, MD DMSc, Anders Rodell, PhD, Kim Vang, PhD, Mallar Chakravarty, PhD, and Ole Munk, PhD, for kind assistances with data acquisition and development of software for analysis at the Department of Nuclear Medicine and PET Center at the Aarhus University Hospital. Supported by grants from the Danish Council for Independent Research 1994-2016.
Funding
This research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
AG: conceived the study, collected the subjects to be analyzed and received the permission to do the experiments. JK: critically evaluated and revised the manuscript. YN: conducted the initial analysis. NHSC: assisted the analysis and contributed to drafting the article. DFW: critically revised the manuscript. AM: supervised the tomography and critically revised the manuscript. All authors have given their final approval to the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This report refers to no studies of animals of which any one of the authors is responsible.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The possible mechanism of the inverted-U changes of binding potentials as functions of age determined in the present study can be ascribed to at least two factors that include the receptor density and the availability of receptors as reflection of the concentration of the endogenous ligand (dopamine). We may ascribe the observation that the ratios of BPND of cortices to putamen differ somewhat from the ratio of receptor densities to differences of extracellular dopamine concentrations between the cerebral cortices and striatum. Major differences of the dopamine concentration can be estimated by the formula for the concentration of a competitor, derived from the definition of the binding potential and maximum binding capacity,
where \({V}_{\mathrm{T}}\) is the total distribution volume of the tracer, \({C}_{\mathrm{i}}\) and \({K}_{\mathrm{i}}\) the steadystate and half-inhibition concentrations of endogenous ligands or other competitors, respectively, and α the availability of the receptors, defined as the fraction of un-occupied receptors. Availability can then be described by following equation,
where \(\sigma\) is the degree of occupancy of the receptors by all competing ligands. The combination of the approximately linear decline of the maximum binding capacity as a function of age, and the inverse U-shape of the relationship between the actual binding potential and age, suggests that two reciprocally active factors are in operation, as also expressed by the rearrangement of Eqs. 1 and 2,
where \({BP}_{0}\) is the theoretically highest achievable binding potential, i.e., the binding potential in the absence of competitors, equal to \({B}_{\mathrm{max}}/({V}_{\mathrm{T}} {K}_{\mathrm{D}}\)). It is possible to assess the concentration of competitors as function of age by regression of Eq. 6 to the inverted U-shape of the relationship between binding potential and age, on the basis of three claims: The maximum binding capacity declines linearly with age [60], the inverted-U shape of the binding potential as function of age dictates a linear increase of the availability of the receptors with age- associated loss of dopamine [55, 61], and the average occupancy by dopamine is 10% at the age of 30 [62], consistent with an availability of 90%. In another study [63], the authors estimated D2/3 receptor occupancy by dopamine to be 21% at an average age of 25, with the assumption that it might be even higher due to an incomplete depletion of synaptic dopamine, after administration of the tyrosine hydroxylase inhibitor alpha-methyl-para-tyrosine (AMPT). Here, we set the occupancy level at the age of 25 at 50% corresponding to an availability of 50%. The resulting regression equation rearranges to,
where \({BP}_{0.25}\) is the value of \({BP}_{0}\) at age 25, \({b}_{\alpha }\) the rate of increase of availability as function of age, A the age of the subject, and \({b}_{\mathrm{BP}}\) the rate of decline of the maximum binding capacity (\({BP}_{0}\)) as function of age. The regression parameter \({b}_{\alpha }\) was chosen to be 0.1, providing the optimal R2 values for the regression to be fitted to the data (see Fig.
Mean R2 values as a function of bα. The R2 is coefficient of determination, which determines convergence of model with experimental data and the higher value of R2 results in higher accuracy, which is seen bα equal 0.1. Abscissa: Log10 rescaling of b values, the rate of increase of availability as function of age
5).
The results of the non-linear regression with Eq. 3 shown in Figs. 1 and 2 include the estimates of dopamine concentrations, calculated from Eq. 3, the decline of BP0, and the estimation of availability as function of age. The values calculated by the ERLiBiRD method were the lowest among those tested by all four methods in all regions, explained by the inclusion of the contents of the vascular volume into the calculation of the quantity of non-displaceable tracer. It is known that the ratio of precision to accuracy of the ERLiBiRD method is higher than in the reference tissue methods of Logan and SRTM [30], as also revealed by the coefficient of variation determined in the present study, but the accuracy is not directly quantifiable without consideration of the volume of the vascular bed and the partition coefficient of the radioligand raclopride. With a partition coefficient of 0.5 [29] and a vascular volume of 5% of the whole-brain volume, inclusion of the radioactivity in the vascular bed in the calculation of the binding potential with ERLiBiRD accounts for 10% in the steady-state, enough to explain the lower binding potentials obtained with the ERLiBiRD method.
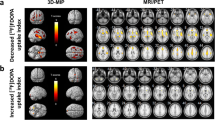
The parametric mapping demonstrated significant foci of age-related decline bilaterally in putamen, in the right insula, and in regions of the temporal cortex (superior temporal gyrus, middle temporal gyrus) and regions of the frontal cortex (precentral gyrus). In the present study, we observed significant declines of values in insula and frontal cortex in the VOI analysis, as well by the parametric mapping analysis. In the putamen of patients with early Parkinson’s disease, D2/3 receptors undergo up-regulation [55], whereas in the prefrontal cortex of individuals with advanced Parkinson’s disease, D2/3 receptors appear to decline [22]. The combination of increased receptor density in the caudate nucleus and decreased values of BPND in the cortex has been reported for patients with schizophrenia [56, 57]. In patients with Parkinson’s disease and schizophrenia, estimates of BPND with [11C]raclopride may therefore reveal changes of dopamine concentrations in the cerebral cortex, as well as in the striatum. Another interesting aspect to investigate would be the comparison between genders, in whom in a recent study, Fazio et al. [58] found a negative correlation between BPND and age, and an effect of gender with higher values of BPND in females [58], perhaps related to period phases. In the present study, because of the comparatively low number of women investigated (one quarter of all subjects tested), exclusion of women from the analysis did not significantly change the results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khodaii, J., Nomura, Y., Chang, N.H.S. et al. Dopamine D2/3 Receptor Availabilities in Striatal and Extrastriatal Regions of the Adult Human Brain: Comparison of Four Methods of Analysis. Neurochem Res 48, 1517–1530 (2023). https://doi.org/10.1007/s11064-022-03825-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03825-4