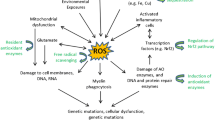
Abstract
Multiple Sclerosis (MS) is a degenerative disorder of the central nervous system (CNS) with complicated etiology that has not been clearly analyzed until nowadays. Apart from anti-inflammatory, immune modulatory and symptomatic treatments, which are the main tools towards MS control, antioxidant molecules may be of interest. Oxidative stress is a key condition implicated in the disease progression. Reactive species production is associated with immune cell activation in the brain as well as in the periphery, accounting for demyelinating and axonal disruptive processes. This review refers to research articles, of the last decade. It describes biological evaluation of antioxidant drugs, and molecules with pharmaceutical interest, which are not designed for MS treatment, however they seem to have potency against MS. Their antioxidant effect is accompanied, in most of the cases, by anti-inflammatory, immune-modulatory and neuroprotective properties. Compounds with such characteristics are expected to be beneficial in the treatment of MS, alone or as complementary therapy, improving some clinical and mechanistic aspects of the disease. This review also summarizes some of the pathobiological characteristics of MS, as well as the role of oxidative stress and inflammation in the progression of neurodegeneration. It presents known drugs and bioactive compounds with antioxidant, and in many cases, pleiotropic activity that have been tested for their efficacy in MS progression or the experimentally induced MS. Antioxidants may offer reduction or prevention of the disease symptoms and progression. Thus, their results may, combined with already applied treatments, be beneficial for the development of new molecules or the repurposing of drugs and supplements that are used with other indication so far.
Graphical Abstract








Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Compston A, Coles A (2008) Multiple sclerosis. The Lancet 372:1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
Pegoretti V, Swanson KA, Bethea JR, Probert L, Eisel ULM, Fischer R (2020) Inflammation and oxidative stress in multiple sclerosis: consequences for therapy development. Oxid Med Cell Longev 2020:7191080. https://doi.org/10.1155/2020/7191080
Henderson AP, Barnett MH, Parratt JD, Prineas JW (2009) Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 66:739–753. https://doi.org/10.1002/ana.21800
Lassmann H (2018) Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol 9:3116. https://doi.org/10.3389/fimmu.2018.03116
Giovannoni G, Tomic D, Bright JR, Havrdová E (2017) “No evident disease activity”: The use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 23:1179–1187. https://doi.org/10.1177/1352458517703193
Khan N, Smith MT (2014) Multiple sclerosis-induced neuropathic pain: pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology 22:1–22. https://doi.org/10.1007/s10787-013-0195-3
Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C et al (2020) Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 77:1132–1140. https://doi.org/10.1001/jamaneurol.2020.1568
Lin CC, Edelson BT (2015) New insights into the role of IL-1β in experimental autoimmune encephalomyelitis and multiple sclerosis. J Immunol 198:4553–4560. https://doi.org/10.4049/jimmunol.1700263
Probert L (2015) TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 302:2–22. https://doi.org/10.1016/j.neuroscience.2015.06.038
Fischer R, Sendetski M, Del Rivero T, Martinez GF, Bracchi-Ricard V, Swanson KA et al (2019) TNFR2 promotes treg-mediated recovery from neuropathic pain across sexes. Proc Natl Acad Sci U S A 116:17045–17050. https://doi.org/10.1073/pnas.1902091116
Neumann B, Segel M, Chalut KJ, Franklin RJ (2019) Remyelination and ageing: reversing the ravages of time. Mult Scler 25:1835–1841. https://doi.org/10.1177/1352458519884006
Heß K, Starost L, Kieran NW, Thomas C, Vincenten MCJ, Antel J et al (2020) Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol 140:359–375. https://doi.org/10.1007/s00401-020-02189-9
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94:329–354. https://doi.org/10.1152/physrev.00040.2012
Ursini F, Maiorino M, Forman HJ (2016) Redox homeostasis: the golden mean of healthy living. Redox Biol 8:205–215. https://doi.org/10.1016/j.redox.2016.01.010
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157–1180. https://doi.org/10.1007/s00204-013-1034-4
Cheng Y, Gulbins E, Siemen D (2011) Activation of the permeability transition pore by Bax via inhibition of the mitochondrial BK channel. Cell Physiol Biochem 27:191–200. https://doi.org/10.1159/000327944
Adamczyk-Sowa M, Galiniak S, Żyracka E, Grzesik M, Naparło K, Sowa P et al (2017) Oxidative modification of blood serum proteins in multiple sclerosis after interferon beta and melatonin treatment. Oxid Med Cell Longev 2017:7905148. https://doi.org/10.1155/2017/7905148
Haider L (2015) Inflammation, iron, energy failure, and oxidative stress in the pathogenesis of multiple sclerosis. Oxid Med Cell Longev 2015:725370. https://doi.org/10.1155/2015/725370
Nikić I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM et al (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 17:495–499. https://doi.org/10.1038/nm.2324
Mossakowski AA, Pohlan J, Bremer D, Lindquist R, Millward JM, Bock M et al (2015) Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol 130:799–814. https://doi.org/10.1007/s00401-015-1497-x
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6:193–201. https://doi.org/10.1038/nrneurol.2010.17
Papagiouvannis G, Theodosis-Nobelos P, Kourounakis PN, Rekka EA (2021) Multi-target directed compounds with antioxidant and/or anti- inflammatory properties as potent agents for alzheimer’s disease. Med Chem 17:1086–1103. https://doi.org/10.2174/1573406416666201013161303
Ohl K, Tenbrock K, Kipp M (2016) Oxidative stress in multiple sclerosis: central and peripheral mode of action. Exp Neurol 277:58–67. https://doi.org/10.1016/j.expneurol.2015.11.010
Kees F (2013) Dimethyl fumarate: a Janus-faced substance? Expert Opin Pharmacother 14:1559–1567. https://doi.org/10.1517/14656566.2013.804912
Nellessen A, Nyamoya S, Zendedel A, Slowik A, Wruck C, Beyer C et al (2020) Nrf2 deficiency increases oligodendrocyte loss, demyelination, neuroinflammation and axonal damage in an MS animal model. Metab Brain Dis 35:353–362. https://doi.org/10.1007/s11011-019-00488-z
Dong Y, D’Mello C, Pinsky W, Lozinski BM, Kaushik DK, Ghorbani S et al (2021) Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat Neurosci 24:489–503. https://doi.org/10.1038/s41593-021-00801-z
Ferreira B, Mendes F, Osório N, Caseiro A, Gabriel A, Valado A (2013) Glutathione in multiple sclerosis. Br J Biomed Sci 70:75–79. https://doi.org/10.1080/09674845.2013.11669939
Li S, Vana AC, Ribeiro R, Zhang Y (2011) Distinct role of nitric oxide and peroxynitrite in mediating oligodendrocyte toxicity in culture and in experimental autoimmune encephalomyelitis. Neuroscience 184:107–119. https://doi.org/10.1016/j.neuroscience.2011.04.007
Ortiz GG, Macías-Islas MA, Pacheco-Moisés FP, Cruz-Ramos JA, Sustersik S, Barba EA, Aguayo A (2009) Oxidative stress is increased in serum from Mexican patients with relapsing-remitting multiple sclerosis. Dis Markers 26:35–39. https://doi.org/10.3233/DMA-2009-0602
Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H (2013) Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 74:848–861. https://doi.org/10.1002/ana.23974
Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA (2005) Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A 102:9936–9941. https://doi.org/10.1073/pnas.0502552102
Rosito M, Testi C, Parisi G, Cortese B, Baiocco P, Di Angelantonio S (2020) Exploring the use of dimethyl fumarate as microglia modulator for neurodegenerative diseases treatment. Antioxidants (Basel) 9:700. https://doi.org/10.3390/antiox9080700
Scuderi SA, Ardizzone A, Paterniti I, Esposito E, Campolo M (2020) Antioxidant and anti-inflammatory effect of Nrf2 inducer dimethyl fumarate in neurodegenerative diseases. Antioxidants (Basel) 9:630. https://doi.org/10.3390/antiox9070630
Yan N, Xu Z, Qu C, Zhang J (2021) Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int Immunopharmacol 98:107844. https://doi.org/10.1016/j.intimp.2021.107844
Shih HJ, Yen JC, Chiu AW, Chow YC, Pan WH, Wang TY, Huang CJ (2015) FTY720 mitigates torsion/detorsion-induced testicular injury in rats. J Surg Res 196:325–331. https://doi.org/10.1016/j.jss.2015.03.014
Yevgi R, Demir R (2021) Oxidative stress activity of fingolimod in multiple sclerosis. Clin Neurol Neurosurg 202:106500. https://doi.org/10.1016/j.clineuro.2021.106500
Colombo E, Di Dario M, Capitolo E, Chaabane L, Newcombe J, Martino G, Farina C (2014) Fingolimod may support neuroprotection via blockade of astrocyte nitric oxide. Ann Neurol 76:325–337. https://doi.org/10.1002/ana.24217
Tasset I, Bahamonde C, Agüera E, Conde C, Cruz AH, Pérez-Herrera A et al (2013) Effect of natalizumab on oxidative damage biomarkers in relapsing-remitting multiple sclerosis. Pharmacol Rep 65:624–631. https://doi.org/10.1016/s1734-1140(13)71039-9
Kipp M (2020) Does siponimod exert direct effects in the central nervous system? Cells 9:1771. https://doi.org/10.3390/cells9081771
Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, EXPAND Clinical Investigators et al (2018) Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet 391:1263–1273. https://doi.org/10.1016/S0140-6736(18)30475-6
Colombo E, Bassani C, De Angelis A, Ruffini F, Ottoboni L, Comi G et al (2020) Siponimod (BAF312) activates Nrf2 while hampering NFκB in human astrocytes, and protects from astrocyte-induced neurodegeneration. Front Immunol 11:635. https://doi.org/10.3389/fimmu.2020.00635
Zhang L, Guo K, Zhou J, Zhang X, Yin S, Peng J et al (2021) Ponesimod protects against neuronal death by suppressing the activation of A1 astrocytes in early brain injury after experimental subarachnoid hemorrhage. J Neurochem 158:880–897. https://doi.org/10.1111/jnc.15457
Pouzol L, Piali L, Bernard CC, Martinic MM, Steiner B, Clozel M (2019) Therapeutic potential of ponesimod alone and in combination with dimethyl fumarate in experimental models of multiple sclerosis. Innov Clin Neurosci 16:22–30
Lassiter G, Melancon C, Rooney T, Murat AM, Kaye JS, Kaye AM et al (2020) Ozanimod to treat relapsing forms of multiple sclerosis: a comprehensive review of disease, drug efficacy and side effects. Neurol Int 12:89–108. https://doi.org/10.3390/neurolint12030016
Fronza M, Lorefice L, Frau J, Cocco E (2021) An overview of the efficacy and safety of ozanimod for the treatment of relapsing multiple sclerosis. Drug Des Devel Ther 15:1993–2004. https://doi.org/10.2147/DDDT.S240861
Cohan S, Kumar J, Arndorfer S, Zhu X, Zivkovic M, Tencer T (2021) Comparative efficacy and safety of ozanimod and dimethyl fumarate for relapsing-remitting multiple sclerosis using matching-adjusted indirect comparison. CNS Drugs 35:795–804. https://doi.org/10.1007/s40263-021-00805-0
Agresti C, Mechelli R, Olla S, Veroni C, Eleuteri C, Ristori G, Salvetti M (2020) Oxidative status in multiple sclerosis and off-targets of antioxidants: the case of edaravone. Curr Med Chem 27:2095–2105. https://doi.org/10.2174/0929867326666190124122752
Bakhtiari M, Ghasemi N, Salehi H, Amirpour N, Kazemi M, Mardani M (2021) Evaluation of Edaravone effects on the differentiation of human adipose derived stem cells into oligodendrocyte cells in multiple sclerosis disease in rats. Life Sci 282:119812. https://doi.org/10.1016/j.lfs.2021.119812
Minnelli C, Laudadio E, Galeazzi R, Rusciano D, Armeni T, Stipa P et al (2019) Synthesis, characterization and antioxidant properties of a new lipophilic derivative of edaravone. Antioxidants (Basel) 8:258. https://doi.org/10.3390/antiox8080258
Faissner S, Mishra M, Kaushik DK, Wang J, Fan Y, Silva C et al (2017) Systematic screening of generic drugs for progressive multiple sclerosis identifies clomipramine as a promising therapeutic. Nat Commun 8:1990. https://doi.org/10.1038/s41467-017-02119-6
Brown D, Moezzi D, Dong Y, Koch M, Yong VW (2021) Combination of hydroxychloroquine and indapamide attenuates neurodegeneration in models relevant to multiple sclerosis. Neurotherapeutics 18:387–400. https://doi.org/10.1007/s13311-020-01002-5
Dziedzic A, Saluk-Bijak J, Miller E, Bijak M (2020) Metformin as a potential agent in the treatment of multiple sclerosis. Int J Mol Sci 21:5957. https://doi.org/10.3390/ijms21175957
Horakova O, Kroupova P, Bardova K, Buresova J, Janovska P, Kopecky J, Rossmeisl M (2019) Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci Rep 9:6156. https://doi.org/10.1038/s41598-019-42531-0
Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S (2009) Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 182:8005–8014. https://doi.org/10.4049/jimmunol.0803563
Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R et al (2016) Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol 292:58–67. https://doi.org/10.1016/j.jneuroim.2016.01.014
Paintlia AS, Paintlia MK, Mohan S, Singh AK, Singh I (2013) AMP-activated protein kinase signaling protects oligodendrocytes that restore central nervous system functions in an experimental autoimmune encephalomyelitis model. Am J Pathol 183:526–541. https://doi.org/10.1016/j.ajpath.2013.04.030
Stevanović I, Ninković M, Stanojević J, Mančić B, Stojanović I (2021) Therapeutic potential of agmatine in the experimental autoimmune encephalomyelitis. Vojnosanit Pregl 78:834–843. https://doi.org/10.2298/VSP190707145S
Chai J, Luo L, Hou F, Fan X, Yu J, Ma W et al (2016) Agmatine reduces lipopolysaccharide-mediated oxidant response via activating PI3K/Akt pathway and up-regulating Nrf2 and HO-1expression in macrophages. PLoS ONE 11:e0163634. https://doi.org/10.1371/journal.pone.0163634
Atalay S, Jarocka-Karpowicz I, Skrzydlewska E (2019) Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants (Basel) 9:21. https://doi.org/10.3390/antiox9010021
Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ (2018) Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem 293:16546–16558. https://doi.org/10.1074/jbc.RA118.004929
Fiani B, Sarhadi KJ, Soula M, Zafar A (2020) Quadri SA (2020) current application of cannabidiol (CBD) in the management and treatment of neurological disorders. Neurol Sci 41(11):3085–3098. https://doi.org/10.1007/s10072-020-04514-2
Kozela E, Juknat A, Kaushansky N, Ben-Nun A, Coppola G, Vogel Z (2015) Cannabidiol, a non-psychoactive cannabinoid, leads to EGR2-dependent anergy in activated encephalitogenic T cells. J Neuroinflammation 12:52. https://doi.org/10.1186/s12974-015-0273-0
Perras C (2005) Sativex for the management of multiple sclerosis symptoms. Issues Emerg Health Technol 72:1–4
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D (2014) Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the guideline development subcommittee of the american academy of neurology. Neurology 82:1556–1563. https://doi.org/10.1212/WNL.0000000000000363
Pedre B, Barayeu U, Ezeriņa D, Dick TP (2021) The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species. Pharmacol Ther 228:107916. https://doi.org/10.1016/j.pharmthera.2021
Bavarsad Shahripour R, Harrigan MR, Alexandrov AV (2014) N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain and behavior 4:108–122. https://doi.org/10.1002/brb3.208
Monti DA, Zabrecky G, Leist TP, Wintering N, Bazzan AJ, Zhan T, Newberg AB (2020) N-acetyl cysteine administration is associated with increased cerebral glucose metabolism in patients with multiple sclerosis: an exploratory study. Front Neurol 11:88. https://doi.org/10.3389/fneur.2020.00088
Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G et al (2013) N-Acetylcysteine boosts brain and blood glutathione in gaucher and parkinson diseases. Clin Neuropharmacol 36:103–106. https://doi.org/10.1097/WNF.0b013e31829ae713
Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB (2010) Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc Natl Acad Sci USA 107:8416–8421. https://doi.org/10.1073/pnas.0910627107
Farfan-Garcia ED, Castillo-Hernandez MC, Pinto-Almazan R, Rivas-Arancibia S, Gallardo JM, Guerra-Araiza C (2014) Tibolone prevents oxidation and ameliorates cholinergic deficit induced by ozone exposure in the male rat hippocampus. Neurochem Res 39:1776–1786. https://doi.org/10.1007/s11064-014-1385-0
Pinto-Almazan R, Segura-Uribe JJ, Soriano-Ursua MA, Farfan-Garcia ED, Gallardo JM, Guerra-Araiza C (2018) Effect of tibolone pretreatment on kinases and phosphatases that regulate the expression and phosphorylation of Tau in the hippocampus of rats exposed to ozone. Neural Regen Res 13:440–448. https://doi.org/10.4103/1673-5374.228726
Mancino DNJ, Lima A, Roig P, García Segura LM, De Nicola AF, Garay LI (2022) Tibolone restrains neuroinflammation in mouse experimental autoimmune encephalomyelitis. J Neuroendocrinol 34:e13078. https://doi.org/10.1111/jne.13078
Valerio M, Liu HB, Heffner R, Zivadinov R, Ramanathan M, Weinstock-Guttman B, Awad AB (2011) Phytosterols ameliorate clinical manifestations and inflammation in experimental autoimmune encephalomyelitis. Inflamm Res 60:457–465. https://doi.org/10.1007/s00011-010-0288-z
Baig MW, Nasir B, Waseem D, Majid M, Khan MZI, Haq IU (2020) Withametelin: a biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm J 28:1526–1537. https://doi.org/10.1016/j.jsps.2020.09.021
Khan A, Shal B, Khan AU, Bibi T, Islam SU, Baig MW et al (2021) Withametelin, a novel phytosterol, alleviates neurological symptoms in EAE mouse model of multiple sclerosis via modulation of Nrf2/HO-1 and TLR4/NF-κB signaling. Neurochem Int 151:105211. https://doi.org/10.1016/j.neuint.2021.105211
Gutiérrez-Miranda B, Gallardo I, Melliou E, Cabero I, Álvarez Y, Magiatis P et al (2020) Oleacein attenuates the pathogenesis of experimental autoimmune encephalomyelitis through both antioxidant and anti-inflammatory effects. Antioxidants (Basel) 9:1161. https://doi.org/10.3390/antiox9111161
Michaličková D, Hrnčíř T, Canová NK, Slanař O (2020) Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur J Pharmacol 873:172973. https://doi.org/10.1016/j.ejphar.2020.172973
Jesus M, Martins AP, Gallardo E, Silvestre S (2016) Diosgenin: recent highlights on pharmacology and analytical methodology. J Anal Methods Chem 2016:4156293. https://doi.org/10.1155/2016/4156293
Xiao L, Guo D, Hu C, Shen W, Shan L, Li C et al (2012) Diosgenin promotes oligodendrocyte progenitor cell differentiation through estrogen receptor-mediated ERK1/2 activation to accelerate remyelination. Glia 60(7):1037–1052
Zeinali H, Baluchnejadmojarad T, Roghani M (2021) Diosgenin ameliorates cellular and molecular changes in multiple sclerosis in C57BL/6 mice. Mult Scler Relat Disord 55:103211. https://doi.org/10.1016/j.msard.2021.103211
Ramos-Hryb AB, Pazini FL, Kaster MP, Rodrigues ALS (2017) Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs 31:1029–1041. https://doi.org/10.1007/s40263-017-0474-4
Yamamoto S, Sakemoto C, Iwasa K, Maruyama K, Shimizu K, Yoshikawa K (2020) Ursolic acid treatment suppresses cuprizone-induced demyelination and motor dysfunction via upregulation of IGF-1. J Pharmacol Sci 144:119–122. https://doi.org/10.1016/j.jphs.2020.08.002
Ye P, Li L, Richards RG, DiAugustine RP, D’Ercole AJ (2002) Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci 22:6041–6051. https://doi.org/10.1523/JNEUROSCI.22-14-06041.2002
Zhang Y, Li X, Ciric B, Curtis MT, Chen WJ, Rostami A, Zhang GX (2020) A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination. Proc Natl Acad Sci U S A 117:9082–9093. https://doi.org/10.1073/pnas.2000208117
Emamgholipour S, Hossein-Nezhad A, Sahraian MA, Askarisadr F, Ansari M (2016) Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci 145:34–41. https://doi.org/10.1016/j.lfs.2015.12.014
Farez MF, Mascanfroni ID, Méndez-Huergo SP, Yeste A, Murugaiyan G, Garo LP et al (2015) Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 162:1338–1352. https://doi.org/10.1016/j.cell.2015.08.025
Rathnasamy G, Ling EA, Kaur C (2014) Therapeutic implications of melatonin in cerebral edema. Histol Histopathol 29:1525–1538. https://doi.org/10.14670/HH-29.1525
Miller E, Walczak A, Majsterek I, Kędziora J (2013) Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol 257:97–101. https://doi.org/10.1016/j.jneuroim.2013.02.012
Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A (2012) Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci 314:37–40. https://doi.org/10.1016/j.jns.2011.11.003
Lucas RM, Byrne SN, Correale J, Ilschner S, Hart PH (2015) Ultraviolet radiation, vitamin D and multiple sclerosis. Neurodegener Dis Manag 5:413–424. https://doi.org/10.2217/nmt.15.33
Munger KL, Åivo J, Hongell K, Soilu-Hänninen M, Surcel HM, Ascherio A (2016) Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the finnish maternity cohort. JAMA Neurol 73:515–519. https://doi.org/10.1001/jamaneurol.2015.4800
Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR et al (2011) A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 77:1611–1618. https://doi.org/10.1212/WNL.0b013e3182343274
Hart PH, Jones AP, Trend S, Cha L, Fabis-Pedrini MJ, Cooper MN et al (2018) A randomised, controlled clinical trial of narrowband UVB phototherapy for clinically isolated syndrome: The PhoCIS study. Mult Scler J Exp Transl Clin 4:2055217318773112. https://doi.org/10.1177/2055217318773112
Oliveira SR, Simão ANC, Alfieri DF, Flauzino T, Kallaur AP, Mezzaroba L et al (2017) Vitamin D deficiency is associated with disability and disease progression in multiple sclerosis patients independently of oxidative and nitrosative stress. J Neurol Sci 381:213–219. https://doi.org/10.1016/j.jns.2017.07.046
Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P (2012) Vitamin D as a protective factor in multiple sclerosis. Neurology 79:2140–2145. https://doi.org/10.1016/j.jns.2017.07.046
Mayne CG, Spanier JA, Relland LM, Williams CB, Hayes CE (2011) 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol 41:822–832. https://doi.org/10.1002/eji.201040632
Cantorna MT, Snyder L, Lin YD, Yang L (2015) Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 7:3011–3021. https://doi.org/10.3390/nu7043011
Yang M, Qin Z, Zhu Y (2013) Vitamin D-binding protein in cerebrospinal fluid is associated with multiple sclerosis progression. Mol Neurobiol 47:946–956. https://doi.org/10.1007/s12035-012-8387-1
Sandberg L, Biström M, Salzer J, Vågberg M, Svenningsson A, Sundström P (2016) Vitamin D and axonal injury in multiple sclerosis. Mult Scler 22:1027–1031. https://doi.org/10.1177/1352458515606986
Soilu-Hänninen M, Aivo J, Lindström BM, Elovaara I, Sumelahti ML, Färkkilä M et al (2012) A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 83:565–571. https://doi.org/10.1136/jnnp-2011-301876
Rotstein DL, Healy BC, Malik MT, Carruthers RL, Musallam AJ, Kivisakk P et al (2015) Effect of vitamin D on MS activity by disease-modifying therapy class. Neurol Neuroimmunol Neuroinflamm 2:e167. https://doi.org/10.1212/NXI.0000000000000167
Khosravi-Largani M, Pourvali-Talatappeh P, Rousta AM, Karimi-Kivi M, Noroozi E, Mahjoob A et al (2018) A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci 10:37–44. https://doi.org/10.1016/j.ensci.2018.01.007
Taş S, Sarandöl E, Dirican M (2014) Vitamin B6 supplementation improves oxidative stress and enhances serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. Sci World J. https://doi.org/10.1155/2014/351598
Prakhova LN, Ilves AG, Savintceva ZI, Mineev KK, Nikiforova IG, Rubanik KS, Kataeva GV (2016) Neuroprotection therapy of multiple sclerosis with high doses of ethylmethylhydroxypyridine succinate. Zh Nevrol Psikhiatr Im S S Korsakova 116:73–78. https://doi.org/10.17116/jnevro201611610273-78
Moghaddasi M, Mamarabadi M, Mohebi N, Razjouyan H, Aghaei M (2013) Homocysteine, vitamin B12 and folate levels in Iranian patients with multiple sclerosis: a case control study. Clin Neurol Neurosurg 115:1802–1805. https://doi.org/10.1016/j.clineuro.2013.05.007
Zhu Y, He ZY, Liu HN (2011) Meta-analysis of the relationship between homocysteine, vitamin B12, folate, and multiple sclerosis. J Clin Neurosci 18:933–938. https://doi.org/10.1016/j.jocn.2010.12.022
Nozari E, Ghavamzadeh S, Razazian N (2019) The effect of vitamin B12 and folic acid supplementation on serum homocysteine, anemia status and quality of life of patients with multiple sclerosis. Clin Nutr Res 8:36–45. https://doi.org/10.7762/cnr.2019.8.1.36
Shang Z, Cai W, Cao Y, Wang F, Wang Z, Lu J, Zhang J (2017) An integrated strategy for rapid discovery and identification of the sequential piperine metabolites in rats using ultra high-performance liquid chromatography/high resolution mass spectrometery. J Pharm Biomed Anal 146:387–401. https://doi.org/10.1016/j.jpba.2017.09.012
Mittal R, Gupta RL (2000) In vitro antioxidant activity of piperine. Methods Find Exp Clin Pharmacol 22:271–274. https://doi.org/10.1358/mf.2000.22.5.796644
Roshanbakhsh H, Elahdadi Salmani M, Dehghan S, Nazari A, Javan M, Pourabdolhossein F (2020) Piperine ameliorated memory impairment and myelin damage in lysolecethin induced hippocampal demyelination. Life Sci 253:117671. https://doi.org/10.1016/j.lfs.2020.117671
Fletcher JL, Murray SS, Xiao J (2018) Brain-derived neurotrophic factor in central nervous system myelination: a new mechanism to promote myelin plasticity and repair. Int J Mol Sci 19:4131. https://doi.org/10.3390/ijms19124131
Ikram M, Ullah R, Khan A, Kim MO (2020) Ongoing research on the role of gintonin in the management of neurodegenerative disorders. Cells 9:1464. https://doi.org/10.3390/cells9061464
Choi JH, Oh J, Lee MJ, Ko SG, Nah SY, Cho IH (2021) Gintonin mitigates experimental autoimmune encephalomyelitis by stabilization of Nrf2 signaling via stimulation of lysophosphatidic acid receptors. Brain Behav Immun 93:384–398. https://doi.org/10.1016/j.bbi.2020.12.004
Schmitz K, Brunkhorst R, de Bruin N, Mayer CA, Häussler A, Ferreiros N et al (2017) Dysregulation of lysophosphatidic acids in multiple sclerosis and autoimmune encephalomyelitis. Acta Neuropathol Commun 5:42. https://doi.org/10.1186/s40478-017-0446-4
Li W, Zhang Z, Zhang K, Xue Z, Li Y, Zhang Z et al (2016) Arctigenin suppress Th17 cells and ameliorates experimental autoimmune encephalomyelitis through AMPK and PPAR-γ/ROR-γt signaling. Mol Neurobiol 53:5356–5366. https://doi.org/10.1186/s40478-017-0446-4
Kim TW, Kim Y, Jung W, Kim DE, Keum H, Son Y, Jon S (2021) Bilirubin nanomedicine ameliorates the progression of experimental autoimmune encephalomyelitis by modulating dendritic cells. J Control Release 331:74–84. https://doi.org/10.1016/j.jconrel.2021.01.019
Ferreira GC, McKenna MC (2017) L-carnitine and acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem Res 42:1661–1675. https://doi.org/10.1007/s11064-017-2288-7
Assaf N, Shalby AB, Khalil WK, Ahmed HH (2012) Biochemical and genetic alterations of oxidant/antioxidant status of the brain in rats treated with dexamethasone: protective roles of melatonin and acetyl-L-carnitine. J Physiol Biochem 68:77–90. https://doi.org/10.1007/s13105-011-0121-3
Zidan A, Hedya SE, Elfeky DM, Abdin AA (2018) The possible anti-apoptotic and antioxidant effects of acetyl l-carnitine as an add-on therapy on a relapsing-remitting model of experimental autoimmune encephalomyelitis in rats. Biomed Pharmacother 103:1302–1311. https://doi.org/10.1016/j.biopha.2018.04.173
Rajda C, Bergquist J, Vécsei L (2007) Kynurenines, redox disturbances and neurodegeneration in multiple sclerosis. J Neural Transm Suppl 72:323–329. https://doi.org/10.1007/978-3-211-73574-9_40
Biernacki T, Sandi D, Bencsik K, Vécsei L (2020) Kynurenines in the pathogenesis of multiple sclerosis: therapeutic perspectives. Cells 9:1564. https://doi.org/10.3390/cells9061564
Majlath Z, Annus A, Vecsei L (2018) Kynurenine system and multiple sclerosis, pathomechanism and drug targets with an emphasis on laquinimod. Curr Drug Targets 19:805–814. https://doi.org/10.2174/1389450117666161223125417
Ruffini F, Rossi S, Bergamaschi A, Brambilla E, Finardi A, Motta C et al (2013) Laquinimod prevents inflammation-induced synaptic alterations occurring in experimental autoimmune encephalomyelitis. Mult Scler 19:1084–1094. https://doi.org/10.1177/1352458512469698
Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, Filippi M, ALLEGRO Study Group (2012) Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 366:1000–1009. https://doi.org/10.1056/NEJMoa1104318
Theodosis-Nobelos P, Papagiouvannis G, Rekka EA (2021) A Review on vitamin E natural analogues and on the design of synthetic vitamin E derivatives as cytoprotective agents. Mini Rev Med Chem 21:10–22. https://doi.org/10.2174/1389557520666200807132617
Khalilian B, Madadi S, Fattahi N, Abouhamzeh B (2021) Coenzyme Q10 enhances remyelination and regulate inflammation effects of cuprizone in corpus callosum of chronic model of multiple sclerosis. J Mol Histol 52:125–134. https://doi.org/10.1007/s10735-020-09929-x
Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Jazayeri S, Reza Gohari M (2013) Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int J Neurosci 123:776–782. https://doi.org/10.3109/00207454.2013.801844
Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P (2018) Coenzyme Q10 supplementation in aging and disease. Front Physiol 9:44. https://doi.org/10.3389/fphys.2018.00044
Mao P, Manczak M, Shirendeb UP, Reddy PH (2013) MitoQ, a mitochondria-targeted antioxidant, delays disease progression and alleviates pathogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Biochim Biophys Acta 1832:2322–2331. https://doi.org/10.1016/j.bbadis.2013.09.005
Avetisyan AV, Samokhin AN, Alexandrova IY, Zinovkin RA, Simonyan RA, Bobkova NV (2016) Mitochondrial dysfunction in neocortex and hippocampus of olfactory bulbectomized mice, a model of alzheimer’s disease. Biochemistry (Mosc) 81:615–623. https://doi.org/10.1134/S0006297916060080
Fetisova EK, Muntyan MS, Lyamzaev KG, Chernyak BV (2019) Therapeutic Effect of the mitochondria-targeted antioxidant SkQ1 on the culture model of multiple sclerosis. Oxid Med Cell Longev 2019:2082561. https://doi.org/10.1155/2019/2082561
Nesterenko AM, Kholina EG, Lyamzaev KG, Mulkidjanian AY, Chernyak BV (2019) Molecular dynamics modeling of the interaction of cationic fluorescent lipid peroxidation-sensitive probes with the mitochondrial membrane. Dokl Biochem Biophys 486:220–223. https://doi.org/10.1134/S1607672919030153
Vorobjeva N, Prikhodko A, Galkin I, Pletjushkina O, Zinovkin R, Sud’ina G et al (2017) Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur J Cell Biol 96:254–265. https://doi.org/10.1016/j.ejcb.2017.03.003
Sousa BC, Pitt AR, Spickett CM (2017) Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic Biol Med 111:294–308. https://doi.org/10.1016/j.freeradbiomed.2017.02.003
Bispo VS, de Arruda Campos IP, Di Mascio P, Medeiros MH (2016) Structural elucidation of a carnosine-acrolein adduct and its quantification in human urine samples. Sci Rep 6:19348. https://doi.org/10.1038/srep19348
Colzani M, De Maddis D, Casali G, Carini M, Vistoli G, Aldini G (2016) Reactivity, selectivity, and reaction mechanisms of aminoguanidine, hydralazine, pyridoxamine, and carnosine as sequestering agents of reactive carbonyl species: a comparative study. ChemMedChem 11:1778–1789. https://doi.org/10.1002/cmdc.201500552
Regazzoni L, de Courten B, Garzon D, Altomare A, Marinello C, Jakubova M et al (2016) A carnosine intervention study in overweight human volunteers: bioavailability and reactive carbonyl species sequestering effect. Sci Rep 6:27224. https://doi.org/10.1038/srep27224
Chmielewska K, Dzierzbicka K, Inkielewicz-Stępniak I, Przybyłowska M (2020) Therapeutic potential of carnosine and its derivatives in the treatment of human diseases. Chem Res Toxicol 33:1561–1578. https://doi.org/10.1021/acs.chemrestox.0c00010
Jukić I, Kolobarić N, Stupin A, Matić A, Kozina N, Mihaljević Z et al (2021) Carnosine, small but mighty-prospect of use as functional ingredient for functional food formulation. Antioxidants (Basel) 10:1037. https://doi.org/10.3390/antiox10071037
Spaas J, Franssen WMA, Keytsman C, Blancquaert L, Vanmierlo T, Bogie J et al (2021) Carnosine quenches the reactive carbonyl acrolein in the central nervous system and attenuates autoimmune neuroinflammation. J Neuroinflammation 18:255. https://doi.org/10.1186/s12974-021-02306-9
Simicic J, Ostojić S (2019) Medium-term carnosine supplementation positively affects patient-reported outcomes in multiple sclerosis. J Neurol Sci 405:104. https://doi.org/10.1016/j.jns.2019.10.1761
Zanini D, Jezdimirovic T, Stajer V, Ostojic J, Maksimovic N, Ostojic SM (2020) Dietary supplementation with L-carnosine improves patient-reported outcomes, autonomic nervous system performance, and brain metabolism in 3 adult patients with multiple sclerosis. Nutr Res 84:63–69. https://doi.org/10.1016/j.nutres.2020.09.008
Acknowledgements
Not applicable
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: PTN. Analyzed the data: PTN. Wrote the manuscript: PTN. Editing of the manuscript: PTN, EAR. Critically reviewed the article: PTN, EAR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participation
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Theodosis-Nobelos, P., Rekka, E.A. Efforts Towards Repurposing of Antioxidant Drugs and Active Compounds for Multiple Sclerosis Control. Neurochem Res 48, 725–744 (2023). https://doi.org/10.1007/s11064-022-03821-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03821-8