Abstract
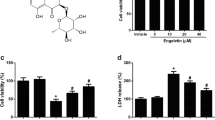
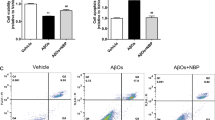
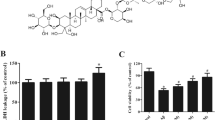
Ferroptosis and neuroinflammation play a crucial role in the pathogenesis of Alzheimer’s disease (AD), and Edaravone (EDA) has been demonstrated to have anti-inflammatory, antioxidant and neuroprotective effects in neurodegenerative diseases. However, the relationship between EDA and ferroptosis in AD is unidentified. This research aimed to elucidate the mechanism of EDA in AD with Aβ 1-42-induced HT22 cells as in vitro cell model. The results showed that EDA could significantly reduce Aβ1-42-induced apoptosis of HT22 cells and formation of pro-inflammatory factors TNF-α, IL-1β and IL-6, prevent the activation of TLR4/NF-κB /NLRP3 signaling pathway, and inhibit ferroptosis and lipid peroxidation. Taken together, EDA contributes to inhibiting neuroinflammatory injury and ferroptosis in Aβ 1-42-induced HT22 cells, and thus may be a potential candidate for the treatment of AD.




Similar content being viewed by others
Data Availability
The datasets used and analysed in the current study are available from the corresponding author on reasonable request.
References
Anand R, Gill K, Mahdi A (2014) Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2013.07.004
Scheltens P, Blennow K, Breteler M, de Strooper B, Frisoni G, Salloway S, Van der Flier W (2016) Alzheimer’s disease. Lancet (London, England) 388:505–517. https://doi.org/10.1016/s0140-6736(15)01124-1
Park J, Kim S, Kim S, Jo M, Choi M, Kim M (2019) A novel kit for early diagnosis of Alzheimer’s disease using a fluorescent nanoparticle imaging. Sci Rep 9:13184. https://doi.org/10.1038/s41598-019-49711-y
Li LJ, Chen ZW, Zhong XF, Huang JF, Yu Q, Carlsson C, Okonkwo O (2019) Sweetening the process of biomarker discovery in Alzheimer’s disease: development of improved chemical strategies for probing glycosylation patterns in AD. In: Abstracts of Papers of The American Chemical Society
Cai Y, Chai Y, Fu Y, Wang Y, Zhang Y, Zhang X, Zhu L, Miao M, Yan T (2021) Salidroside Ameliorates Alzheimer’s disease by targeting NLRP3 inflammasome-mediated pyroptosis. Front Aging Neurosci 13:809433. https://doi.org/10.3389/fnagi.2021.809433
World Alzheimer Report (2021) Alzheimer Diseases International. https://www.alzint.org/resource/world-alzheimer-report-2021/
Emre C, Do K, Jun B, Hjorth E, Alcalde S, Kautzmann M, Gordon W, Nilsson P, Bazan N, Schultzberg M (2021) Age-related changes in brain phospholipids and bioactive lipids in the APP knock-in mouse model of Alzheimer’s disease. Acta Neuropathol Commun 9:116. https://doi.org/10.1186/s40478-021-01216-4
Zhuang W, Yue L, Dang X, Chen F, Gong Y, Lin X, Luo Y (2019) Rhodiola Rosenroot (): potential applications in aging-related diseases. Aging Dis 10:134–146. https://doi.org/10.14336/ad.2018.0511
Mangalmurti A, Lukens J (2022) How neurons die in Alzheimer’s disease: implications for neuroinflammation. Curr Opin Neurobiol 75:102575. https://doi.org/10.1016/j.conb.2022.102575
Sun Y, Xia X, Basnet D, Zheng J, Huang J, Liu J (2022) Mechanisms of ferroptosis and emerging links to the pathology of neurodegenerative diseases. Front Aging Neurosci 14:904152. https://doi.org/10.3389/fnagi.2022.904152
Ayton S, Portbury S, Kalinowski P, Agarwal P, Diouf I, Schneider J, Morris M, Bush A (2021) Regional brain iron associated with deterioration in Alzheimer’s disease: a large cohort study and theoretical significance. Alzheimer’s Dement J Alzheimer’s Assoc 17:1244–1256. https://doi.org/10.1002/alz.12282
Wei Y, Lv H, Shaikh A, Han W, Hou H, Zhang Z, Wang S, Shang P (2020) Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim Biophys Acta Gen Subj 1864:129539. https://doi.org/10.1016/j.bbagen.2020.129539
Yu H, Guo P, Xie X, Wang Y, Chen G (2017) Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med 21:648–657. https://doi.org/10.1111/jcmm.13008
Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35:830–849. https://doi.org/10.1016/j.ccell.2019.04.002
Wen Y, Chen H, Zhang L, Wu M, Zhang F, Yang D, Shen J, Chen J (2021) Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic Biol Med 173:41–51. https://doi.org/10.1016/j.freeradbiomed.2021.07.019
Friedmann Angeli J, Schneider M, Proneth B, Tyurina Y, Tyurin V, Hammond V, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm G, Geissler E, Thomas S, Stockwell B, O’Donnell V, Kagan V, Schick J, Conrad M (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16:1180–1191. https://doi.org/10.1038/ncb3064
Shan Y, Li J, Zhu A, Kong W, Ying R, Zhu W (2022) Ginsenoside Rg3 ameliorates acute pancreatitis by activating the NRF2/HO–1–mediated ferroptosis pathway. Int J Mol Med. https://doi.org/10.3892/ijmm.2022.5144
Xiang Z, Zhou X, Mranda G, Xue Y, Wang Y, Wei T, Liu J, Ding Y (2022) Identification of the ferroptosis-related ceRNA network related to prognosis and tumor immunity for gastric cancer. Aging. https://doi.org/10.18632/aging.204176
Yang S, Xie Z, Pei T, Zeng Y, Xiong Q, Wei H, Wang Y, Cheng W (2022) Salidroside attenuates neuronal ferroptosis by activating the Nrf2/HO1 signaling pathway in Aβ-induced Alzheimer’s disease mice and glutamate-injured HT22 cells. Chin Med 17:82. https://doi.org/10.1186/s13020-022-00634-3
Feng T, Yamashita T, Sasaki R, Tadokoro K, Matsumoto N, Hishikawa N, Abe K (2021) Protective effects of edaravone on white matter pathology in a novel mouse model of Alzheimer’s disease with chronic cerebral hypoperfusion. J Cereb Blood Flow Metab 41:1437–1448. https://doi.org/10.1177/0271678x20968927
Writing G, Edaravone ALSSG (2017) Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 16:505–512. https://doi.org/10.1016/S1474-4422(17)30115-1
Edaravone Acute Infarction Study G (2003) Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis 15:222–229. https://doi.org/10.1159/000069318
Wang H, Zhang T, Huang J, Xiang J, Chen J, Fu J, Zhao Y (2017) Edaravone attenuates the proinflammatory response in amyloid-β-treated microglia by inhibiting NLRP3 inflammasome-mediated IL-1β secretion. Cell Physiol Biochem 43:1113–1125. https://doi.org/10.1159/000481753
Zhang G, Zhang L, Guo Y, Ma Z, Wang H, Li T, Liu J, Du Y, Yao L, Li T, Du J (2017) Protective effect of edaravone against Aβ25-35-induced mitochondrial oxidative damage in SH-SY5Y cells. Cell Mol Biol (Noisy-le-Grand France) 63:36–42. https://doi.org/10.14715/cmb/2017.63.5.8
Zhao X, Huang X, Yang C, Jiang Y, Zhou W, Zheng W (2022) Artemisinin attenuates amyloid-induced brain inflammation and memory impairments by modulating TLR4/NF-kappaB signaling. Int J Mol Sci. https://doi.org/10.3390/ijms23116354
Zhao Z, Luan P, Huang S, Xiao S, Zhao J, Zhang B, Gu B, Pi R, Liu J (2013) Edaravone protects HT22 neurons from H2O2-induced apoptosis by inhibiting the MAPK signaling pathway. CNS Neurosci Ther 19:163–169. https://doi.org/10.1111/cns.12044
Liu J, Yan X, Qi L, Li L, Hu G, Li P, Zhao G (2015) Ginsenoside Rd attenuates Aβ25-35-induced oxidative stress and apoptosis in primary cultured hippocampal neurons. Chemico-Biol Interact 239:12–18. https://doi.org/10.1016/j.cbi.2015.06.030
Shen Y, Wang Y, Yu G, Liu C, Zhang Z, Zhang L (2013) Effects of edaravone on amyloid-β precursor protein processing in SY5Y-APP695 cells. Neurotox Res 24:139–147. https://doi.org/10.1007/s12640-012-9370-3
Zhang Z, Han K, Wang C, Sun C, Jia N (2020) Dioscin protects against Aβ1–42 oligomers-induced neurotoxicity via the function of SIRT3 and autophagy. Chem Pharm Bull 68:717–725. https://doi.org/10.1248/cpb.c20-00046
Sharma P, Srivastava P, Seth A, Tripathi P, Banerjee A, Shrivastava S (2019) Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog Neurobiol 174:53–89. https://doi.org/10.1016/j.pneurobio.2018.12.006
Wang F, Wang J, Shen Y, Li H, Rausch W, Huang X (2022) Iron dyshomeostasis and ferroptosis: a new Alzheimer’s disease hypothesis? Front Aging Neurosci 14:830569. https://doi.org/10.3389/fnagi.2022.830569
Muresan V, Ladescu Muresan Z (2015) Amyloid-β precursor protein: multiple fragments, numerous transport routes and mechanisms. Exp Cell Res 334:45–53. https://doi.org/10.1016/j.yexcr.2014.12.014
Canter R, Penney J, Tsai L (2016) The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 539:187–196. https://doi.org/10.1038/nature20412
Yoon S, Kim YK (2015) The role of immunity and neuroinflammation in genetic predisposition and pathogenesis of Alzheimer’s disease. Aims Genet 2:230–249
Choi A, Ryter S, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368:651–662. https://doi.org/10.1056/NEJMra1205406
Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins I, Rousseau F, Schymkowitz J, De Strooper B (2010) Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J 29:3408–3420. https://doi.org/10.1038/emboj.2010.211
Iijima K, Liu H, Chiang A, Hearn S, Konsolaki M, Zhong Y (2004) Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: a potential model for Alzheimer’s disease. Proc Natl Acad Sci USA 101:6623–6628. https://doi.org/10.1073/pnas.0400895101
Younkin S (1995) Evidence that A beta 42 is the real culprit in Alzheimer’s disease. Ann Neurol 37:287–288. https://doi.org/10.1002/ana.410370303
Zheng M, Liu J, Ruan Z, Tian S, Ma Y, Zhu J, Li G (2013) Intrahippocampal injection of Aβ1–42 inhibits neurogenesis and down-regulates IFN-γ and NF-κB expression in hippocampus of adult mouse brain. Amyloid 20:13–20. https://doi.org/10.3109/13506129.2012.755122
Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R (2010) Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflamm 7:30. https://doi.org/10.1186/1742-2094-7-30
Zhou H, Niu D, Xue B, Li F, Liu X, He Q, Wang X, Wang X (2003) Triptolide inhibits TNF-alpha, IL-1 beta and NO production in primary microglial cultures. NeuroReport 14:1091–1095. https://doi.org/10.1097/01.wnr.0000073682.00308.47
Huang X, Li J, Tao Y, Wang X, Zhang R, Zhang J, Su Z, Huang Q, Deng Y (2018) viaGeniposide attenuates Aβ-induced neurotoxicity the TLR4/NF-κB pathway in HT22 cells. RSC Adv 8:18926–18937. https://doi.org/10.1039/c8ra01038b
Liu M, Xie J, Sun Y (2020) TLR4/MyD88/NF-κB-mediated inflammation contributes to cardiac dysfunction in rats of PTSD. Cell Mol Neurobiol 40:1029–1035. https://doi.org/10.1007/s10571-020-00791-9
Guo H, Callaway J, Ting J (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. https://doi.org/10.1038/nm.3893
Cui W, Sun C, Ma Y, Wang S, Wang X, Zhang Y (2020) Inhibition of TLR4 induces M2 microglial polarization and provides neuroprotection via the NLRP3 inflammasome in Alzheimer’s Disease. Front NeuroSci 14:444. https://doi.org/10.3389/fnins.2020.00444
Zhang B, Lian W, Zhao J, Wang Z, Liu A, Du G (2021) DL0410 alleviates memory impairment in D-galactose-induced aging rats by suppressing neuroinflammation via the TLR4/MyD88/NF-kappaB pathway. Oxid Med Cell Longev 2021:6521146. https://doi.org/10.1155/2021/6521146
Calvo-Rodriguez M, Garcia-Rodriguez C, Villalobos C, Nunez L (2020) Role of toll like receptor 4 in Alzheimer’s disease. Front Immunol 11:1588. https://doi.org/10.3389/fimmu.2020.01588
Funding
None.
Author information
Authors and Affiliations
Contributions
SG and RC wrote the paper and conceived and designed the experiments; QL and HG analyzed the data; QY and YX collected and provided the sample for this study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Lei, Q., Guo, H. et al. Edaravone Attenuates Aβ 1-42-Induced Inflammatory Damage and Ferroptosis in HT22 Cells. Neurochem Res 48, 570–578 (2023). https://doi.org/10.1007/s11064-022-03782-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03782-y