Abstract
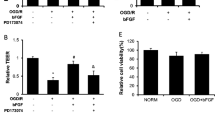
Multiple paracrine factors are implicated in the regulation of barrier properties of human brain endothelial cells (BECs) in different physiologic and pathologic settings. We have recently demonstrated that autocrine secretion of basic fibroblast growth factor (bFGF) by BECs is necessary for the establishment of endothelial barrier (as demonstrated by high trans-endothelial electric resistance, TEER), whereas exogenous bFGF inhibits TEER in a concentration-dependent manner. In the present study we analysed the contribution of MAPK/ERK and STAT3 signalling pathways to the inhibitory effects of exogenous bFGF. Treatment with bFGF (8 ng/ml) for 3 days increased phosphorylation of ERK1/2 and STAT3. Treatment with FGF receptor 1 (FGFR1) inhibitor PD173074 (15 μM) suppressed both basal and bFGF-induced activation of ERK1/2 and STAT3. Suppression of STAT signalling with Janus kinase inhibitor JAKi (15 nM) alone or in the presence of bFGF did not change TEER in BEC monolayers. Exposure to JAKi affected neither proliferation, nor expression and distribution of tight junction (TJ) proteins claudin-5, occludin and zonula occludens-1 (ZO-1). In contrast, treatment with MEK 1/2 inhibitor U0126 (10 μM) partially neutralised inhibitory effect of bFGF thus increasing TEER, whereas U0126 alone did not affect resistance of endothelial barrier. Our findings demonstrate that MAPK/ERK signalling pathway does not affect autocrine bFGF signalling-dependent BECs barrier function but is largely responsible for the disruptive effects of the exogenous bFGF. We speculate that bFGF may (depending on concentration and possibly origin) dynamically regulate permeability of the endothelial blood–brain barrier.






Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pivoriunas A, Verkhratsky A (2021) Astrocyte-endotheliocyte axis in the regulation of the blood–brain barrier. Neurochem Res 46:2538–2550
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood–brain barrier. Cell 163:1064–1078
Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) blood–brain barrier: from physiology to disease and back. Physiol Rev 99:21–78
Semyanov A, Verkhratsky A (2021) Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci 44:781–792
Vardjan N, Parpura V, Verkhratsky A, Zorec R (2019) Gliocrine system: astroglia as secretory cells of the CNS. Adv Exp Med Biol 1175:93–115
Alvarez JI, Katayama T, Prat A (2013) Glial influence on the blood brain barrier. Glia 61:1939–1958
Neal EH, Marinelli NA, Shi Y, McClatchey PM, Balotin KM, Gullett DR, Hagerla KA, Bowman AB, Ess KC, Wikswo JP, Lippmann ES (2019) A simplified, fully defined differentiation scheme for producing blood–brain barrier endothelial cells from human iPSCs. Stem Cell Rep 12:1380–1388
Kriauciunaite K, Pociute A, Kausyle A, Pajarskiene J, Verkhratsky A, Pivoriunas A (2021) Concentration-dependent duality of bFGF in regulation of barrier properties of human brain endothelial cells. J Cell Physiol 236:7642–7654
Ornitz DM, Itoh N (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev 4:215–266
Chi X, Tai HH (2010) Interleukin-4 up-regulates 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in human lung cancer cells. Exp Cell Res 316:2251–2259
Galoczova M, Coates P, Vojtesek B (2018) STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett 23:12
Turkson J, Bowman T, Adnane J, Zhang Y, Djeu JY, Sekharam M, Frank DA, Holzman LB, Wu J, Sebti S, Jove R (1999) Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol 19:7519–7528
Chung J, Uchida E, Grammer TC, Blenis J (1997) STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol 17:6508–6516
Haq R, Halupa A, Beattie BK, Mason JM, Zanke BW, Barber DL (2002) Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J Biol Chem 277:17359–17366
Lim CP, Cao X (1999) Serine phosphorylation and negative regulation of Stat3 by JNK. J Biol Chem 274:31055–31061
Jain N, Zhang T, Kee WH, Li W, Cao X (1999) Protein kinase C delta associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem 274:24392–24400
Kim JH, Yoon MS, Chen J (2009) Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J Biol Chem 284:35425–35432
Gonzalez-Mariscal L, Tapia R, Chamorro D (2008) Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778:729–756
Fischer S, Wiesnet M, Renz D, Schaper W (2005) H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol 84:687–697
Wang LF, Li X, Gao YB, Wang SM, Zhao L, Dong J, Yao BW, Xu XP, Chang GM, Zhou HM, Hu XJ, Peng RY (2015) Activation of VEGF/Flk-1-ERK pathway induced blood–brain barrier injury after microwave exposure. Mol Neurobiol 52:478–491
Takata F, Dohgu S, Matsumoto J, Machida T, Sakaguchi S, Kimura I, Yamauchi A, Kataoka Y (2018) Oncostatin M-induced blood–brain barrier impairment is due to prolonged activation of STAT3 signaling in vitro. J Cell Biochem 119:9055–9063
Takata F, Dohgu S, Sakaguchi S, Sakai K, Yamanaka G, Iwao T, Matsumoto J, Kimura I, Sezaki Y, Tanaka Y, Yamauchi A, Kataoka Y (2019) Oncostatin-m-reactive pericytes aggravate blood–brain barrier dysfunction by activating JAK/STAT3 signaling in vitro. Neuroscience 422:12–20
Kolczynska K, Loza-Valdes A, Hawro I, Sumara G (2020) Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: a review. Lipids Health Dis 19:113
Fessler E, Borovski T, Medema JP (2015) Endothelial cells induce cancer stem cell features in differentiated glioblastoma cells via bFGF. Mol Cancer 14:157
Yang D, Li Z, Gao G, Li X, Liao Z, Wang Y, Li W, Zhang Y, Liu W (2021) Combined analysis of surface protein profile and microRNA expression profile of exosomes derived from brain microvascular endothelial cells in early cerebral ischemia. ACS Omega 6:22410–22421
Nakamura K, Arimura K, Nishimura A, Tachibana M, Yoshikawa Y, Makihara N, Wakisaka Y, Kuroda J, Kamouchi M, Ooboshi H, Kitazono T, Ago T (2016) Possible involvement of basic FGF in the upregulation of PDGFRbeta in pericytes after ischemic stroke. Brain Res 1630:98–108
Shimizu F, Sano Y, Abe MA, Maeda T, Ohtsuki S, Terasaki T, Kanda T (2011) Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J Cell Physiol 226:255–266
Linnerbauer M, Rothhammer V (2020) Protective functions of reactive astrocytes following central nervous system insult. Front Immunol 11:573256
Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Diaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Gotz M, Gutierrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai KK, Norris CM, Okada S, Oliet SHR, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Perez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein JD, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24:312–325
Xie C, Shen X, Xu X, Liu H, Li F, Lu S, Gao Z, Zhang J, Wu Q, Yang D, Bao X, Zhang F, Wu S, Lv Z, Zhu M, Xu D, Wang P, Cao L, Wang W, Yuan Z, Wang Y, Li Z, Teng H, Huang Z (2020) Astrocytic YAP promotes the formation of glia scars and neural regeneration after spinal cord injury. J Neurosci 40:2644–2662
Sofroniew MV (2020) Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol 41:758–770
Funding
This research has received funding from European Regional Development Fund (Project No 13.1.1-LMT-K-718-05-0005) under grant agreement with the Research Council of Lithuania (LMTLT). Funded as European Union’s measure in response to Cov-19 pandemic.
Author information
Authors and Affiliations
Contributions
Author Contributions: Conceptualization, A.P., A.V.; Investigation, K.K., A.P., A.K.; Formal analysis, K.K., A.P., A.K; Art work, K.K., A.P., A.V; Writing – original draft, K.K., A.P., A.V; Writing – review & editing, A.P., A.V.; Supervision, A.P., A.V.; Funding acquisition, A.P., A.V.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kriaučiūnaitė, K., Pociūtė, A., Kaušylė, A. et al. Basic Fibroblast Growth Factor Opens and Closes the Endothelial Blood–Brain Barrier in a Concentration-Dependent Manner. Neurochem Res 48, 1211–1221 (2023). https://doi.org/10.1007/s11064-022-03678-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03678-x