Abstract
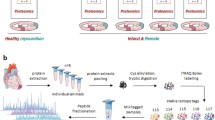
Myocardial infraction (MI) is the principal risk factor for the onset of heart failure (HF). Investigations regarding the physiopathology of MI progression to HF have revealed the concerted engagement of other tissues, such as the autonomic nervous system and the medulla oblongata (MO), giving rise to systemic effects, important in the regulation of heart function. Cardiac sympathetic afferent denervation following application of resiniferatoxin (RTX) attenuates cardiac remodelling and restores cardiac function following MI. While the physiological responses are well documented in numerous species, the underlying molecular responses during the initiation and progression from MI to HF remains unclear. We obtained multi-tissue time course proteomics with a murine model of HF induced by MI in conjunction with RTX application. We isolated tissue sections from the left ventricle (LV), MO, cervical spinal cord and cervical vagal nerves at four time points over a 12-week study. Bioinformatic analyses consistently revealed a high statistical enrichment for metabolic pathways in all tissues and treatments, implicating a central role of mitochondria in the tissue-cellular response to both MI and RTX. In fact, the additional functional pathways found to be enriched in these tissues, involving the cytoskeleton, vesicles and signal transduction, could be downstream of responses initiated by mitochondria due to changes in neuronal pulse frequency after a shock such as MI or the modification of such frequency communication from the heart to the brain after RTX application. Development of future experiments, based on our proteomic results, should enable the dissection of more precise mechanisms whereby metabolic changes in neuronal and cardiac tissues can effectively ameliorate the negative physiological effects of MI via RTX application.





Similar content being viewed by others
Data availability
The data that support the findings from this study are available from the corresponding author upon reasonable request.
References
Savarese G, Division of Cardiology, Department of Medicine, Karolinska Insitutet, Stockholm, Sweden, Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden et al (2017) Global public health burden of heart failure. Cardiac Fail Rev 3:7. https://doi.org/10.15420/cfr.2016:25:2
Arif M, Klevstig M, Benfeitas R et al (2021) Integrative transcriptomic analysis of tissue-specific metabolic crosstalk after myocardial infarction. eLife 10:e66921. https://doi.org/10.7554/eLife.66921
Doran S, Arif M, Lam S et al (2021) Multi-omics approaches for revealing the complexity of cardiovascular disease. Brief Bioinform 22:bbab061. https://doi.org/10.1093/bib/bbab061
Katz SD (2018) Pathophysiology of chronic systolic heart failure. A view from the periphery. Ann ATS 15:S38–S41. https://doi.org/10.1513/AnnalsATS.201710-789KV
Muqtadar H, Testai FD, Gorelick PB (2012) The dementia of cardiac disease. Curr Cardiol Rep 14:732–740. https://doi.org/10.1007/s11886-012-0304-8
Amare AT, Schubert KO, Klingler-Hoffmann M et al (2017) The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies. Transl Psychiatry 7:e1007. https://doi.org/10.1038/tp.2016.261
Doehner W, Ural D, Haeusler KG et al (2018) Heart and brain interaction in patients with heart failure: overview and proposal for a taxonomy. A position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association: heart and brain interaction in heart failure. Eur J Heart Fail 20:199–215. https://doi.org/10.1002/ejhf.1100
Tahsili-Fahadan P, Geocadin RG (2017) Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res 120:559–572. https://doi.org/10.1161/CIRCRESAHA.116.308446
Park CM, Williams ED, Chaturvedi N et al (2017) Associations between left ventricular dysfunction and brain structure and function: findings from the SABRE (Southall and Brent Revisited) Study. JAHA. https://doi.org/10.1161/JAHA.116.004898
Suzuki H, Sumiyoshi A, Matsumoto Y et al (2015) Structural abnormality of the hippocampus associated with depressive symptoms in heart failure rats. NeuroImage 105:84–92. https://doi.org/10.1016/j.neuroimage.2014.10.040
Ovsenik A, Podbregar M, Fabjan A (2021) Cerebral blood flow impairment and cognitive decline in heart failure. Brain Behav. https://doi.org/10.1002/brb3.2176
Tromp J, Westenbrink BD, Ouwerkerk W et al (2018) Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 72:1081–1090. https://doi.org/10.1016/j.jacc.2018.06.050
Elam M, Sverrisdottir YB, Rundqvist B et al (2003) Pathological sympathoexcitation: how is it achieved? Acta Physiol Scand 177:405–411. https://doi.org/10.1046/j.1365-201X.2003.01080.x
May CN, Frithiof R, Hood SG et al (2010) Specific control of sympathetic nerve activity to the mammalian heart and kidney: control of cardiac and renal sympathetic nerve activity. Exp Physiol 95:34–40. https://doi.org/10.1113/expphysiol.2008.046342
Zhang DY, Anderson AS (2014) The sympathetic nervous system and heart failure. Cardiol Clin 32:33–45. https://doi.org/10.1016/j.ccl.2013.09.010
Despas F, Detis N, Dumonteil N et al (2009) Excessive sympathetic activation in heart failure with chronic renal failure: role of chemoreflex activation. J Hypertens 27:1849–1854. https://doi.org/10.1097/HJH.0b013e32832e8d0f
Despas F, Lambert E, Vaccaro A et al (2012) Peripheral chemoreflex activation contributes to sympathetic baroreflex impairment in chronic heart failure. J Hypertens 30:753–760. https://doi.org/10.1097/HJH.0b013e328350136c
Franchitto N, Despas F, Labrunee M et al (2013) Cardiorenal anemia syndrome in chronic heart failure contributes to increased sympathetic nerve activity. Int J Cardiol 168:2352–2357. https://doi.org/10.1016/j.ijcard.2013.01.023
Kishi T (2016) Deep and future insights into neuromodulation therapies for heart failure. J Cardiol 68:368–372. https://doi.org/10.1016/j.jjcc.2016.05.010
Kingma JG, Simard D, Rouleau JR (2017) Influence of cardiac nerve status on cardiovascular regulation and cardioprotection. WJC 9:508. https://doi.org/10.4330/wjc.v9.i6.508
Zahner MR, Li D-P, Chen S-R, Pan H-L (2003) Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551:515–523. https://doi.org/10.1113/jphysiol.2003.048207
Coote JH, Chauhan RA (2016) The sympathetic innervation of the heart: important new insights. Auton Neurosci 199:17–23. https://doi.org/10.1016/j.autneu.2016.08.014
Chen W-W, Xiong X-Q, Chen Q et al (2015) Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol 213:778–794. https://doi.org/10.1111/apha.12447
Yoshie K, Rajendran PS, Massoud L et al (2020) Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight 5:e124477. https://doi.org/10.1172/jci.insight.124477
Danielle S, Jasdeep K, Sala-Mercardo JA et al (2017) Pharmacological cardiac sympathetic afferent denervation in pacing-induced heart failure. FASEB J. https://doi.org/10.1096/fasebj.31.1_supplement.844.2
Wang H-J, Wang W, Cornish KG et al (2014) Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 64:745–755. https://doi.org/10.1161/HYPERTENSIONAHA.114.03699
Kermorgant M, Ben Salem J, Iacovoni JS et al (2021) Cardiac sensory afferents modulate susceptibility to anxio-depressive behaviour in a mouse model of chronic heart failure. Acta Physiol. https://doi.org/10.1111/apha.13601
Foreman RD (1999) Mechanisms of cardiac pain. Annu Rev Physiol 61:143–167. https://doi.org/10.1146/annurev.physiol.61.1.143
Cechetto DF (2014) Cortical control of the autonomic nervous system: cortical autonomic control. Exp Physiol 99:326–331. https://doi.org/10.1113/expphysiol.2013.075192
Wu Y, Hu Z, Wang D et al (2020) Resiniferatoxin reduces ventricular arrhythmias in heart failure via selectively blunting cardiac sympathetic afferent projection into spinal cord in rats. Eur J Pharmacol 867:172836. https://doi.org/10.1016/j.ejphar.2019.172836
Capilupi MJ, Kerath SM, Becker LB (2020) Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb Perspect Med 10:a034173. https://doi.org/10.1101/cshperspect.a034173
Dusi V, De Ferrari GM, Mann DL (2020) Cardiac sympathetic-parasympathetic interaction. JACC Basic Transl Sci 5:811–814. https://doi.org/10.1016/j.jacbts.2020.07.004
Bibevski S, Dunlap ME (2011) Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev 16:129–135. https://doi.org/10.1007/s10741-010-9190-6
Li M, Zheng C, Kawada T et al (2019) Chronic vagal nerve stimulation exerts additional beneficial effects on the beta-blocker-treated failing heart. J Physiol Sci 69:295–303. https://doi.org/10.1007/s12576-018-0646-0
Liu J-J, Huang N, Lu Y et al (2015) Improving vagal activity ameliorates cardiac fibrosis induced by angiotensin II: in vivo and in vitro. Sci Rep 5:17108. https://doi.org/10.1038/srep17108
Zannad F, De Ferrari GM, Tuinenburg AE et al (2015) Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J 36:425–433. https://doi.org/10.1093/eurheartj/ehu345
Chen EY, Tan CM, Kou Y et al (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform 14:128. https://doi.org/10.1186/1471-2105-14-128
Kuleshov MV, Jones MR, Rouillard AD et al (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44:W90–W97. https://doi.org/10.1093/nar/gkw377
Xie Z, Bailey A, Kuleshov MV et al (2021) Gene set knowledge discovery with Enrichr. Curr Protoc. https://doi.org/10.1002/cpz1.90
Pullen AB, Kain V, Serhan CN, Halade GV (2020) Molecular and cellular differences in cardiac repair of male and female mice. JAHA. https://doi.org/10.1161/JAHA.119.015672
Brar TK (2015) Effect of different phases of menstrual cycle on heart rate variability (HRV). JCDR. https://doi.org/10.7860/JCDR/2015/13795.6592
Mena F, Benoit L (2019) Molecular programs underlying differences in the expression of mood disorders in males and females. Brain Res 1719:89–103. https://doi.org/10.1016/j.brainres.2019.05.016
Wang H-J, Rozanski GJ, Zucker IH (2017) Cardiac sympathetic afferent reflex control of cardiac function in normal and chronic heart failure states: cardiac sympathetic afferent reflex control. J Physiol 595:2519–2534. https://doi.org/10.1113/JP273764
Yoshie K, Rajendran PS, Massoud L et al (2018) Cardiac vanilloid receptor-1 afferent depletion enhances stellate ganglion neuronal activity and efferent sympathetic response to cardiac stress. Am J Physiol Heart Circ Physiol 314:H954–H966. https://doi.org/10.1152/ajpheart.00593.2017
The M, MacCoss MJ, Noble WS, Käll L (2016) Fast and accurate protein false discovery rates on large-scale proteomics data sets with percolator 3.0. J Am Soc Mass Spectrom 27:1719–1727. https://doi.org/10.1007/s13361-016-1460-7
Orsburn BC (2021) Proteome discoverer—a community enhanced data processing suite for protein informatics. Proteomes 9:15. https://doi.org/10.3390/proteomes9010015
Smith CL, Eppig JT (2009) The mammalian phenotype ontology: enabling robust annotation and comparative analysis. WIREs Syst Biol Med 1:390–399. https://doi.org/10.1002/wsbm.44
R Foundation for Statistical Computing R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing R Core Team, Vienna
Gao C-H, Yu G, Cai P (2021) ggVennDiagram: an intuitive, easy-to-use, and highly customizable R package to generate Venn diagram. Front Genet 12:706907. https://doi.org/10.3389/fgene.2021.706907
Wickham H (2009) ggplot2. Springer, New York
Jassal B, Matthews L, Viteri G et al (2019) The reactome pathway knowledgebase. Nucleic Acids Res. https://doi.org/10.1093/nar/gkz1031
Ashburner M, Ball CA, Blake JA et al (2000) Gene Ontology: tool for the unification of biology. Nat Genet 25:25–29. https://doi.org/10.1038/75556
The Gene Ontology Consortium, Carbon S, Douglass E et al (2021) The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res 49:D325–D334. https://doi.org/10.1093/nar/gkaa1113
Kolde R (2019) pheatmap: pretty heatmaps
Li D, Mabrouk OS, Liu T et al (2015) Asphyxia-activated corticocardiac signaling accelerates onset of cardiac arrest. Proc Natl Acad Sci USA 112:E2073–E2082. https://doi.org/10.1073/pnas.1423936112
Sapio MR, Neubert JK, LaPaglia DM et al (2018) Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons. J Clin Investig 128:1657–1670. https://doi.org/10.1172/JCI94331
Jeffry JA, Yu S-Q, Sikand P et al (2009) Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS ONE 4:e7021. https://doi.org/10.1371/journal.pone.0007021
Hong J, Lisco AM, Rudebush TL et al (2020) Identification of cardiac expression pattern of transient receptor potential vanilloid type 1 (TRPV1) receptor using a transgenic reporter mouse model. Neurosci Lett 737:135320. https://doi.org/10.1016/j.neulet.2020.135320
Zhao S, Dai Y, Ning X et al (2021) Vagus nerve stimulation in early stage of acute myocardial infarction prevent ventricular arrhythmias and cardiac remodeling. Front Cardiovasc Med 8:648910. https://doi.org/10.3389/fcvm.2021.648910
Doehner W, Frenneaux M, Anker SD (2014) Metabolic impairment in heart failure. J Am Coll Cardiol 64:1388–1400. https://doi.org/10.1016/j.jacc.2014.04.083
Rosano GM, Department of Medical Sciences, IRCCS San Raffaele Pisana, Rome, Italy, Vitale C, Department of Medical Sciences, IRCCS San Raffaele Pisana, Rome, Italy (2018) Metabolic modulation of cardiac metabolism in heart failure. Cardiac Fail Rev 4:99. https://doi.org/10.15420/cfr.2018.18.2
Zucker IH, Xiao L, Haack KKV (2014) The central renin–angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci 126:695–706. https://doi.org/10.1042/CS20130294
Philippou A, Xanthis D, Chryssanthopοulos C et al (2020) Heart failure-induced skeletal muscle wasting. Curr Heart Fail Rep 17:299–308. https://doi.org/10.1007/s11897-020-00468-w
Jahng JWS, Song E, Sweeney G (2016) Crosstalk between the heart and peripheral organs in heart failure. Exp Mol Med 48:e217. https://doi.org/10.1038/emm.2016.20
Van Linthout S, Tschöpe C (2017) Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep 14:251–265. https://doi.org/10.1007/s11897-017-0337-9
Kemp CD, Conte JV (2012) The pathophysiology of heart failure. Cardiovasc Pathol 21:365–371. https://doi.org/10.1016/j.carpath.2011.11.007
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407. https://doi.org/10.1038/s41569-018-0007-y
Fuller GG, Kim JK (2021) Compartmentalization and metabolic regulation of glycolysis. J Cell Sci 134:jcs258469. https://doi.org/10.1242/jcs.258469
Franzoso M, Zaglia T, Mongillo M (2016) Putting together the clues of the everlasting neuro-cardiac liaison. Biochem Biophys Acta 1863:1904–1915. https://doi.org/10.1016/j.bbamcr.2016.01.009
Pius-Sadowska E, Machaliński B (2021) Pleiotropic activity of nerve growth factor in regulating cardiac functions and counteracting pathogenesis. ESC Heart Fail 8:974–987. https://doi.org/10.1002/ehf2.13138
Méloux A, Béjot Y, Rochette L et al (2020) Brain-heart interactions during ischemic processes: clinical and experimental evidences. Stroke 51:679–686. https://doi.org/10.1161/STROKEAHA.119.027732
Miller MA, Zachary JF (2017) Mechanisms and morphology of cellular injury, adaptation, and death. Pathologic basis of veterinary disease. Elsevier, Amsterdam, pp 2-43.e19
Moore AS, Holzbaur EL (2018) Mitochondrial-cytoskeletal interactions: dynamic associations that facilitate network function and remodeling. Curr Opin Physiol 3:94–100. https://doi.org/10.1016/j.cophys.2018.03.003
Constantinides C, Mean R, Janssen BJ (2011) Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J 52:e21-31
Janssen BJA, De Celle T, Debets JJM et al (2004) Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287:H1618–H1624. https://doi.org/10.1152/ajpheart.01192.2003
Acknowledgements
We acknowledge core support from Animal facility ANEXPLO, CREFRE US006 Rangueil, and in particular Xavier Sudre for his expertise. This work was funded by the Foundation de France Grant Number RAF18002BBA awarded to Dina N. Arvanitis. The proteomic investigations were funded by the National Sciences and Engineering Research Council of Canada (F. Beaudry discovery Grant No. RGPIN-2020-05228). Laboratory equipment was funded by the Canadian Foundation for Innovation (CFI) and the Fonds de Recherche du Québec (FRQ), the Government of Quebec (F. Beaudry CFI John R. Evans Leaders Grant No. 36706). F. Beaudry is the holder of the Canada Research Chair in metrology of bioactive molecule and target discovery (Grant No. CRC-2021-00160). This research was undertaken, partly, thanks to funding from the Canada Research Chairs Program. Ph.D. scholarships were awarded to J. Ben Salem from the Fonds de Recherche du Québec—Santé (Scholarship No. 302490) and from the Université de Montréal.
Funding
This work was supported by Foundation de France (Grant No. RAF18002BBA), National Sciences and Engineering Research Council of Canada (Grant No. RGPIN-2020-05228), Canadian Foundation for Innovation (Grant No. 36706), Canada Research Chairs (Grant No. CRC-2021-00160).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salem, J.B., Iacovoni, J.S., Calise, D. et al. Proteomics Reveals Long-Term Alterations in Signaling and Metabolic Pathways Following Both Myocardial Infarction and Chemically Induced Denervation. Neurochem Res 47, 2416–2430 (2022). https://doi.org/10.1007/s11064-022-03636-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03636-7