Abstract
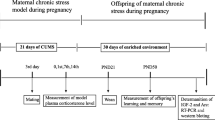
Long-term consequences of stress intervene in normal signaling of the brain leading to many psychological complications. The enriched environment (EE) may potentially ameliorate the stress response in rats. However, the mechanistic understanding of the enriched environment in protecting the myelin membrane from oxidative damage after prolonged exposure to immobilization stress (IS) remains vague. In the current study, we examined the impact of EE by exposing the rats to IS (4 h/day) followed by EE treatment (2 h/day) for 28 days and the activities of ROS, lipid peroxides, and phospholipids were studied, and its influence on the myelin regulatory factor (MyRF) and enzymes linked to sphingolipid was assessed in the forebrain region of myelin membrane. The ROS and lipid peroxidation was increased, and a significant decrease in the antioxidant activities was found in the IS group. IS + EE could reduce oxidative damage and increase the levels of antioxidant activities. The individual phospholipids including sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidic acid (PA) were decreased in the IS group, while IS + EE exhibited significant increase in the phospholipid classes regardless of the exposure to IS. There was down-regulation in the mRNA levels of MyRF, CERS2, SPLTC2, UGT8, and GLTP, while IS + EE could mitigate the up-regulation in the levels of mRNA of MyRF, CERS2, SPLTC2, UGT8, and GLTP. The protein expression of MOG, PLP1, and mTOR was found to be reduced in the IS group of rats, however, IS + EE revealed significant increase in the expression of these signaling molecules. These results suggest that EE had a positive effect on chronic stress response by protecting the myelin membrane against oxidative damage and increasing the protein synthesis required for myelin membrane plasticity via activation of MyRF and mTOR signaling in the forebrain region of IS exposed rats.






Similar content being viewed by others
Abbreviations
- CERS:
-
Ceramide synthase
- EE:
-
Enriched environment
- GLTP:
-
Glycolipid transfer protein
- IS:
-
Immobilization stress
- MOG:
-
Myelin oligodendrocyte glycoprotein
- mTOR:
-
Mammalian target of rapamycin
- MyRF:
-
Myelin regulatory factor
- PLP:
-
Proteolipid protein
- ROS:
-
Reactive oxygen species
- SPLTC:
-
Serine palmitoyltransferase
- UGT:
-
UDP glycosyltransferase
References
Pinto V, Costa JC, Morgado P, Mota C, Miranda A, Bravo FV, Sousa N (2015) Differential impact of chronic stress along the hippocampal dorsal–ventral axis. Brain Struct Funct 220(2):1205–1212
Mariotti A (2015) The effects of chronic stress on health: new insights into the molecular mechanisms of brain–body communication. Future Sci 1:3
Rahman MM, Kerskens CM, Chattarji S, O’Mara SM (2014) Chronic immobilization stress occludes in vivo cortical activation in an animal model of panic induced by carbon dioxide inhalation. Front Behav Neurosci 8:311
Thamizhoviya G, Vanisree AJ (2019) Enriched environment modulates behavior, myelination and augments molecules governing the plasticity in the forebrain region of rats exposed to chronic immobilization stress. Metabolic brain disease 34(3):875–887
Oliveira TG, Chan RB, Bravo FV, Miranda A, Silva RR, Zhou B, Sousa N (2016) The impact of chronic stress on the rat brain lipidome. Mol Psychiatry 21(1):80–88
Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, Becker KA (2013) Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs. Nat Med 19(7):934–938
Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG (2009) Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 34(13):2699–2709
Ravera S, Bartolucci M, Cuccarolo P, Litamè E, Illarcio M, Calzia D, Panfoli I (2015) Oxidative stress in myelin sheath: the other face of the extramitochondrial oxidative phosphorylation ability. Free Radic Res 49(9):1156–1164
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30(1):11–26
Salzer JL (2015) Schwann cell myelination. Cold Spring Harb Perspect Biol 7(8):a020529
Poitelon Y, Kopec AM, Belin S (2020) Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells 9(4):812
Snaidero N, Simons M (2014) Myelination at a glance. J Cell Sci 15(14):2999–3004
Min Y, Kristiansen K, Boggs JM, Husted C, Zasadzinski JA, Israelachvili J (2009) Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proc Natl Acad Sci 106(9):3154–3159
Wood TL, Bercury KK, Cifelli SE, Mursch LE, Min J, Dai J, Macklin WB (2013) mTOR: a link from the extracellular milieu to transcriptional regulation of oligodendrocyte development. ASN Neuro 5(1):AN20120092
Tyler WA, Gangoli N, Gokina P, Kim HA, Covey M, Levison SW, Wood TL (2009) Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci 29(19):6367–6378
Forbes TA, Gallo V (2017) All wrapped up: environmental effects on myelination. Trends Neurosci 40(9):572–587
Bindu B, Rekha J, Kutty BM (2005) Post insult enriched housing improves the 8-arm radial maze performance but not the Morris water maze task in ventral subicular lesioned rats. Brain Res 1063(2):121–131
Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L (2005) Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behavioural Brain Res 163(1):78–90
Rizzi S, Bianchi P, Guidi S, Ciani E, Bartesaghi R (2011) Impact of environmental enrichment on neurogenesis in the dentate gyrus during the early postnatal period. Brain Res 1415:23–33
Johansson BB, Belichenko PV (2002) Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab 22(1):89–96
Xu H, Gelyana E, Rajsombath M, Yang T, Li S, Selkoe D (2016) Environmental enrichment potently prevents microglia-mediated neuroinflammation by human amyloid β- protein oligomers. J Neurosci 36(35):9041–9056
Adlard PA, Perreau VM, Pop V, Cotman CW (2005) Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci 25(17):4217–4221
Herring A, Yasin H, Ambrée O, Sachser N, Paulus W, Keyvani K (2008) Environmental enrichment counteracts Alzheimer’s neurovascular dysfunction in TgCRND8 mice. Brain Pathol 18(1):32–39
Forbes TA, Goldstein EZ, Dupree JL, Jablonska B, Scafidi J, Adams KL, Gallo V (2020) Environmental enrichment ameliorates perinatal brain injury and promotes functional white matter recovery. Nat Commun 11(1):1–17
Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS (2008) Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci 122(2):282–292
Schneider T, Turczak J, Przewlocki R (2006) Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacology 31:36–46
Norton WT, Poduslo SE (1973) Myelination in rat brain: method of myelin isolation. J Neurochem 21(4):749–757
Das NP, Ratty AK (1987) Studies on the effects of the narcotic alkaloids, cocaine, morphine, and codeine on nonenzymatic lipid peroxidation in rat brain mitochondria. Biochem Med Metab Biol 37(2):258–264
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85(2):337–341
Beutler E (1982) Catalase. Red cell metabolism A manual of biochemical methods, 3rd edn. Grune and Stratton, New York, pp 105–106
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Eryomin VA, Poznyakov SP (1989) Quantitative determination of phospholipids using the dyes Victoria blue R and B. Anal Biochem 180(1):186–191
Stewart JC (1980) Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 104(1):10–14
Bongarzone ER, Pasquini JM, Soto EF (1995) Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. J Neurosci Res 41(2):213–221
Chia LS, Thompson JE, Moscarello MA (1983) Disorder in human myelin induced by superoxide radical: an in vitro investigation. Biochem Biophys Res Commun 117(1):141–146
Ortiz GG, Pacheco-Moisés FP, Bitzer-Quintero OK, Ramírez-Anguiano AC, Flores-Alvarado LJ, Ramírez-Ramírez V, Torres-Sánchez ED (2013) Immunology and oxidative stress in multiple sclerosis: clinical and basic approach. Clinical and developmental immunology
Gomez-Cabrera MC, Domenech E, Viña J (2008) Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44(2):126–131
Cui L, Hofer T, Rani A, Leeuwenburgh C, Foster TC (2009) Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol Aging 30(6):903–909
Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Butterfield DA (2008) Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging 29(1):51–70
Herring A, Blome M, Ambrée O, Sachser N, Paulus W, Keyvani K (2010) Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol 20(1):166–175
Radak Z, Marton O, Nagy E, Koltai E, Goto S (2013) The complex role of physical exercise and reactive oxygen species on brain. J Sport Health Sci 2(2):87–93
Lamaziere A, Richard D, Barbe U, Kefi K, Bausero P, Wolf C, Visioli F (2011) Differential distribution of DHA-phospholipids in rat brain after feeding: a lipidomic approach. Prostagland Leukotrien Essential Fatty Acids 84(1–2):7–11
Sato Y, Bernier F, Suzuki I, Kotani S, Nakagawa M, Oda Y (2013) Comparative lipidomics of mouse brain exposed to enriched environment. J Lipid Res 54(10):2687–2696
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Thompson WJ (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28(1):264–278
Bujalka H, Koenning M, Jackson S, Perreau VM, Pope B, Hay CM, Srinivasan R (2013) MYRF is a membrane-associated transcription factor that autoproteolytically cleaves to directly activate myelin genes. PLoS Biol 11(8):e1001625
Duncan GJ, Plemel JR, Assinck P, Manesh SB, Muir FGW, Hirata R (2017) Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol 134(3):403–422
Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA (2009) Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138(1):172–185
McKenzie IA, Ohayon D, Li H, Richardson WD (2014) Motor skill learning requires active central myelination. Science 17(346):318–322
Keiner S, Niv F, Neumann S, Steinbach T, Schmeer C, Hornung K, Redecker C (2017) Effect of skilled reaching training and enriched environment on generation of oligodendrocytes in the adult sensorimotor cortex and corpus callosum. BMC Neurosci 18(1):1–13
Davis DL, Mahawar U, Pope VS, Allegood J, Sato-Bigbee C, Wattenberg BW (2020) Dynamics of sphingolipids and the serine palmitoyltransferase complex in rat oligodendrocytes during myelination. J Lipid Res 61(4):505–522
Yang F, Guan Y, Feng X, Rolfs A, Schlüter H, Luo J (2019) Proteomics of the corpus callosum to identify novel factors involved in hypomyelinated Niemann-Pick Type C disease mice. Mol Brain 12(1):1–11
Imgrund S, Hartmann D, Farwanah H, Eckhardt M, Sandhoff R, Degen J, Willecke K (2009) Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem 284(48):33549–33560
Dupree JL, Suzuki K, Popko B (1998) Galactolipids in the formation and function of the myelin sheath. Microscopy research technique 41(5):431–440
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47(4):379–391
Harrington EP, Zhao CO, Fancy SPJ, Kaing S, Franklin RJM et al (2010) Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol 68:703–716
Narayanan SP, Flores AI, Wang F, Macklin WB (2009) Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci 29:6860–6870
Grier MD, West KL, Kelm ND, Fu C, Does MD, Parker B, Carson RP (2017) Loss of mTORC2 signaling in oligodendrocyte precursor cells delays myelination. PLoS ONE 12(11):e0188417
Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE (2018) Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci 21(5):696–706
Funding
The authors thank the University Grants Commission-Special Assistance Programme, New Delhi, India, for their financial support in the form of research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All the institutional and national guidelines for the care and welfare of the laboratory animals were followed (IAEC No:02/15/2017). The ethical approval was obtained as per the norms of IAEC (Institutional Animal Ethical Clearance-205/GO/ReBi/SL/2000/CPCSEA).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thamizhoviya, G., Vanisree, A.J. Enriched Environment Enhances the Myelin Regulatory Factor by mTOR Signaling and Protects the Myelin Membrane Against Oxidative Damage in Rats Exposed to Chronic Immobilization Stress. Neurochem Res 46, 3314–3324 (2021). https://doi.org/10.1007/s11064-021-03433-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03433-8