Abstract
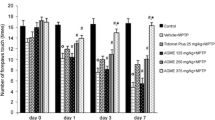
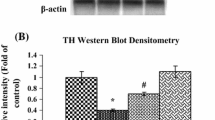
3,4-Dihydroxyphenyl ethanol, known as hydroxytyrosol (HTy), is a phenylpropanoid found in diverse vegetable species. Several studies have demonstrated that HTy is a potent antioxidant. Thus, our study is aimed to evaluate the antioxidant effect of HTy and its derivatives, hydroxytyrosol acetate (HTyA) and nitrohydroxytyrosol (HTyN), in a model of oxidative stress induced by 1-methyl-4-phenylpyridinium (MPP+) in rats. Rats were administered intravenously (i.v.) in the tail with 1 mL saline solution or polyphenol compound (1.5 mg/kg) 5 min before intrastriatal infusion of 10 µg MPP+/8 µL. We found that rats injured with MPP+, pretreatment with HTy, HTyA or HTyN significantly decreased ipsilateral turns. This result was consistent with a significant preservation of striatal dopamine levels and decreased lipid fluorescence products (LFP), a marker of oxidative stress. Brain GSH/GSSG ratio, from rats pretreated with HTy or HTyN showed a significant preservation of that marker, decreased as a consequence of MPP+-induced oxidative damage. These results show an antioxidant effect of HTy, HTyA and HTyN in the MPP+ model of Parkinson’s disease in the rat.





Similar content being viewed by others
Data Availability
All data generated or used during the study are available from the corresponding author by request.
Code Availability
Codes related to the study are available from the corresponding author by request.
References
Clark C (2004) Neuroprotection and pharmacotherapy for motor symptoms in Parkinson’s disease. Lancet Neurol 3:466–474. https://doi.org/10.1016/S1474-4422(04)00823-3
Gómez-Chavarín M, Roldan-Roldan G, Morales-Espinosa G, Pérez-Soto G (2012) Mecanismos fisiopatológicos involucrados en la enfermedad de Parkinson. Arch Neurocien (Mex) 17:25–33
Davis G, Williams A, Markey S, Ebert M, Caine E, Reichert C, Kopin I (1979) Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psych Res 1:249–254. https://doi.org/10.1016/0165-1781(79)90006-4
Pérez-Barrón GA, Avila-Acevedo JG, García-Bores AM, Montes S, García-Jiménez S, León-Rivera I, Rubio-Osornio M, Monroy-Noyola A (2015) Neuroprotective effect of Buddleja cordata methanolic extract in the 1-methyl-4-phenylpyridinium Parkinson’s disease rat model. J Nat Med 69:86–93. https://doi.org/10.1007/s11418-014-0866-4
D’Angelo S, Manna C, Migliardi V, Mazzoni O, Morrica P, Capasso G, Pontoni G, Galletti P, Zappia V (2001) Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab Dispos 29:1492–1498
Gallardo E, Palma-Valdés R, Espartero JL, Santiago M (2014) In vivo striatal measurement of hydroxytyrosol, and its metabolite (homovanillic alcohol), compared with its derivative nitrohydroxytyrosol. Neurosci Lett 579:173–176. https://doi.org/10.1016/j.neulet.2014.07.037
Miro-Casas E, Covas MI, Farre M, Fito M, Ortuno J, Weinbrenner T, Roset P, de la Torre R (2003) Hydroxytyrosol disposition in humans. Clin Chem 49:945–952. https://doi.org/10.1373/49.6.945
Gallardo E, Palma-Valdés R, Sarriá B, Gallardo I, Mateos R, Espartero JL (2006) Synthesis and antioxidant activity of alkyl nitroderivatives of hydroxytyrosol. Molecules 21:656. https://doi.org/10.3390/molecules21050656
Vissers MN, Zock PL, Roodenburg AJC, Leenen R, Katan MB (2002) Olive oil phenols are absorbed in humans. J Nutr 132:409–417. https://doi.org/10.1093/jn/132.3.409
Covas MI, de la Torre K, Farre-Albaladejo M, Kaikkonen J, Fito M, Lopez-Sabater C, Pujadas-Bastardes MA, Joglar J, Weinbrenner T, Lamuela-Raventós RM, de la Torre R (2006) Postpandrial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic Biol Med 40:608–616. https://doi.org/10.1016/j.freeradbiomed.2005.09.027
Christian MS, Sharper VA, Hoberman AM et al (2004) The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug Chem Toxicol 27:309–330. https://doi.org/10.1081/dct-200039714
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health”(ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033, https://doi.org/10.2903/j.efsa.2011.2033. http://www.efsa.europa.eu/efsajournal
Rodriguez-Gutiérrez G, Duthie GG, Wood S, Morrice P, Nicol F, Reid M, Cantlay LL, Kelder T, Horgan GW, Fernández-Bolaños J, Guzmán BD, Roos, (2012) Alperujo extract, hydroxytyrosol, and 3,4-dihidroxyphenylglycol are bioavailable and have antioxidant properties in vitamin E deficient rats-a proteomics and network analysis approach. Mol Nutr Food Res 56:1131–1147. https://doi.org/10.1002/mnfr.201100808
De La Cruz JP, Ruiz-Moreno MI, Guerrero A, Reyes JJ, Benitez-Guerrero A, Espartero JL, González-Correa JA (2015) Differences in the neuroprotective effect of orally administered virgin olive oil (Olea europaea) Polyphenols tyrosol and hydroxytyrosol in rats. J Agric Food Chem 63:5957–5963. https://doi.org/10.1021/acs.jafc.5b00627
Schaffer S, Podstawa M, Visioli F, Bogani P, Müller WE, Eckert GP (2007) Hydroxytyrosol-rich olive mill wastewater protects brain cells in vitro and ex vivo. J Agric Food Chem 55:5043–5049. https://doi.org/10.1021/jf0703710
González-Correa JA, Muñoz-Marín J, Arrebola MM, Guerrero A, Narbona F, López-Villodres J, De La Cruz JP (2007) Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia–reoxygenation. Lipids 42:921–929. https://doi.org/10.1007/s11745-007-3097-6
Tasset I, Pontes AJ, Hinojosa AJ, De la Torre R, Túnez I (2011) Olive oil reduces oxidative damage in a 3-nitropropionic acid-induced Huntington’s disease-like rat model. Nutr Neurosci 14:106–111. https://doi.org/10.1179/1476830511Y.0000000005
González-Correa JA, Navas MD, López-Villodres J, Trujillo M, Espartero JL, De La Cruz JP (2008) Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia-reoxygenation. Neulet 446:143–146. https://doi.org/10.1016/j.neulet.2008.09.022
Trujillo M, Mateos R, Collantes de Teran L, Espartero JL, Cert R, Jover M, Alcudia F, Bautista J, Cert A, Parrado J (2006) Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J Agric Food Chem 54:3779–3785. https://doi.org/10.1021/jf060520z
Grasso S, Siracusa L, Spataforma C, Renis M, Tringali C (2007) Hydroxytyrosol lipophilic analogues: enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg Chem 35:137–152. https://doi.org/10.1016/j.bioorg.2006.09.003
Mateos R, Domínguez MM, Espartero JL, Cert A (2003) Antioxidant effect of phenolic compounds, R-tocopherol, and other minor components in virgin olive oil. J Agric Food Chem 51:7170–7175. https://doi.org/10.1021/jf034415q
Gallardo E, Madrona A, Palma-Valdés R, Trujillo M, Espartero JL, Santiago M (2014) The effect of hydroxytyrosol and its nitroderivatives on catechol-O-methyl transferase activity in rat striatal tissue. RSC Adv 4:61086–61091. https://doi.org/10.1039/C4RA09872B
Gordin A, Kaakkola S, Teravainen H (2004) Clinical advantages of COMT inhibition with entacapone—a review. J Neural Transm 111:1343–1363. https://doi.org/10.1007/s00702-004-0190-3
Fernández-Bolaños J, Heredia A, Rodríguez G, Rodríguez R, Jiménez A, Gillen R (2005) Methods for obtaining purified hydroxytyrosol from products and by products derived from the olive tree. U.S. 6849,770 B2
Trujillo M, Gallardo E, Madrona A, Bravo L, Sarriá B, González-Correa JA, Mateos R, Espartero JL (2014) Synthesis and antioxidant activity of nitrohydroxytyrosol and its acyl derivatives. Agric Food Chem 62:10297–10303. https://doi.org/10.1021/jf503543x
Napolitano A, Panzella L, Savarese M, Sacchi R, Giudicianni I, Paolillo L, D’Ischia M (2004) Acid-induced structural modifications of unsaturated fatty acids and phenolic olive oil constituents by nitrite ions: a chemical assessment. Chem Res Toxicol 17:1329–1337. https://doi.org/10.1021/tx049880b
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, New York
Schwarcz R, Fuxe K, Agnati LF, Hokfelt T, Coyle JT (1979) Rotational behaviour in rats with unilateral striatal kainic acid le- sions: a behavioural model for studies on intact dopamine receptors. Brain Res 170:485–495. https://doi.org/10.1016/0006-8993(79)90966-1
Alcaraz-Zubeldia M, Rojas P, Boll C, Ríos C (2001) Neuroprotective effect of acute and chronic administration of copper (II) sulfate against MPP+ neurotoxicity in mice. Neurochem Res 26:61–66. https://doi.org/10.1023/a:1007680616056
Triggs WJ, Willmore LJ (1984) In vivo lipid peroxidation in rat brain following intracortical Fe++ injection. J Neurochem 42:976–979
Aguirre-Vidal Y, Monroy-Noyola A, Anaya-Ramos L, Arteaga-Silva M, Mendez-Armenta M, Ostoa-Saloma P, Díaz-Zaragoza M, Morales-Montor J, Ríos C, Montes S (2017) β-Estradiol-3-benzoate confers neuroprotection in Parkinson MPP+ rat model through inhibition of lipid peroxidation. Steroids 126:7–14. https://doi.org/10.1016/j.steroids.2017.08.001
Hafeman D, Sunde R, Hoekstra WG (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104:580–587. https://doi.org/10.1093/jn/104.5.580
Pérez-Barrón GA, Montes S, Rubio-Osornio M, Avila-Acevedo JG, García-Jiménez S, Rios LC, Monroy-Noyola A (2020) Hydroxytyrosol inhibits MAO isoforms and prevents neurotoxicity inducible by MPP+ in vivo. Front Biosci (Schol Ed) 12:25–37
Miró-Casas E, Covas MI, Fitó M, Farré-Albadalejo M, Marrugat J, de la Torre R (2003) Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur J Clin Nutr 57:186–190. https://doi.org/10.1038/sj.ejcn.1601532
De la Torre-Carbot K, Chávez-Servín JL, Jaúregui O, Castellote AI, Lamuela-Raventós RM, Fitó M, Covas MI, Muñoz-Aguayo D, López-Sabater MC (2007) Presence of virgin olive oil phenolic metabolites in human low density lipoprotein fraction: Determination by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry. Anal Chim Acta 583:402–410. https://doi.org/10.1016/j.aca.2006.10.029
Visioli F, Poli A, Galli C (2002) Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev 22:65–75. https://doi.org/10.1002/med.1028
Visioli F, Galli C, Grande S et al (2003) Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J Nutr 133:2612–2615. https://doi.org/10.1093/jn/133.8.2612
Vissers MN, Zock PL, Roodenburg AJC, Leenen R, Katan MB (2002) Olive oil phenols are absorbed in humans. J Nutr. https://doi.org/10.1093/jn/132.3.409
Konishi Y, Kobayashi S (2004) Transepithelial transport of chlor-ogenic acid, caffeic acid, and their colonic metabolites in intestinalcaco-2 cell monolayers. J Agric Food Chem 52:2518–2526. https://doi.org/10.1021/jf035407c
Watanabe H, Yashiro T, Tohjo Y, Konishi Y (2006) Non-involvement of the human monocarboxylic acid transporter 1(MCT1) in the transport of phenolic acid. Biosci Biotechnol Biochem 70:928–1933. https://doi.org/10.1271/bbb.60093
Bai C, Yan X, Takenaga M, Sekiya K, Nagata T (1998) Determination of synthetic hydroxytyrosol in rat plasma by CG-MS. J Agric Food Chem 46:3998–4001
Gordon GH, Paiva-Martins F, Almeida M (2001) Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J Agric Food Chem 49:2480–2485. https://doi.org/10.1021/jf000537w
Rodriguez-Morató J, Xicota L, Fitó M, Farré M, Dierssen M, De la Torre R (2015) Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 20:4655–4680. https://doi.org/10.3390/molecules20034655
Blum D, Torch S, Lambeng N, Nissou M, Benabid A, Sadoul R, Verna M (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172. https://doi.org/10.1016/s0301-0082(01)00003-x
Visioli F, Bellomo A, Galli C (1998) Free radical-scavenging properties of olive oil polyphenols. Biochem Biophys Res Commun 247:60–64. https://doi.org/10.1006/bbrc.1998.8735
Zou X, Feng Z, Li Y, Wang Y, Wertz K, Weber P, Fu Y, Liu J (2012) Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: activation of Nrf2 and JNK-p62/SQSTM1 pathways. J Nutr Biochem 23:994–1006. https://doi.org/10.1016/j.jnutbio.2011.05.006
Leri M, Scuto M, Ontario ML, Calabrese V, Calabrese EJ, Bucciantini M, Stefani M (2020) Healthy effects of plant polyphenols: molecular mechanisms. Int J Mol Sci 21(4):1250. https://doi.org/10.3390/ijms21041250
González-Santiago M, Fonollá J, Lopez-Huertas E (2010) Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol Res 61:364–370. https://doi.org/10.1016/j.phrs.2009.12.016
Trovato Salinaro A, Cornelius C, Koverech G, Koverech A, Scuto M, Lodato F, Fronte V, Muccilli V, Reibaldi M, Longo A et al (2014) Cellular stress response, redox status, and vitagenes in glaucoma: a systemic oxidant disorderlinked to Alzheimer’s disease. Front Pharmacol 6(5):129. https://doi.org/10.3389/fphar.2014.00129
Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13(11):1763–811. https://doi.org/10.1089/ars.2009.3074
Zhu L, Liu Z, Feng Z, Hao J, Shen W, Li X, Sun L, Sharman E, Wang Y, Wertz K et al (2010) Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epitelial cells. J Nutr Biochem 21:1089–1098. https://doi.org/10.1016/j.jnutbio.2009.09.006
Martín MA, Ramos S, Granado-Serrano AB, Rodríguez-Ramiro M, Trujillo M, Bravo L, Goya L (2010) Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol Nutr Food Res 54:956–966. https://doi.org/10.1002/mnfr.200900159
Sgarbossa A, Dal Bosco M, Pressi G, Cuzzocrea S, Dal Toso R, Menegazzi M (2012) Phenylpropanoid glycosides from plant cell cultures induce heme oxygenase 1 gene expression in a human keratinocyte cell line by affecting the balance of NRF2 and BACH1 transcription factors. Chem Biol Interact 199:87–95. https://doi.org/10.1016/j.cbi.2012.06.006
Bayram B, Ozcelik B, Grimm S, Roeder T, Schrader C, Ernst IM, Wagner AE, Grune T, Frank J, Rimbach GA (2012) A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res 15:71–81. https://doi.org/10.1089/rej.2011.1245
Yu G, Deng A, Tang W, Ma J, Yuan C, Ma J (2016) Hydroxytyrosol induces phase II detoxifying enzyme expression and effectively protects dopaminergic cells against dopamine- and 6-hydroxydopamine induced cytotoxicity. Neurochem Int 96:113–120. https://doi.org/10.1016/j.neuint.2016.03.005
Funakohi-Tago M, Sakata T, Fujiwara S, Sakakura A, Sugai T, Tago K, Tamura H (2018) Hydroxytyrosol butyrate inhibits 6-OHDA-induced apoptosis through activation of the Nrf2/HO-axis in SH-SY5Y cells. Eur J Pharmacol 834:246–256. https://doi.org/10.1016/j.ejphar.2018.07.043
Zheng A, Li H, Xu J, Cao K, Li H, Pu W, Yang Z, Peng Y, Long J, Liu J et al (2015) Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: role of AMP-activated protein kinase activation. Br J Nutr 113(11):1667–1676. https://doi.org/10.1017/S0007114515000884
Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione, iron and Pakinson’s disease. Biochem Pharmacol 64:1037–1048. https://doi.org/10.1016/s0006-2952(02)01174-7
Stokes AH, Hastings TG, Vrana KE (1999) Cytotoxic and genotoxic potential of dopamine. J Neurosci Res 55:659–665. https://doi.org/10.1002/(SICI)1097-4547(19990315)55:6%3c659::AID-JNR1%3e3.0.CO;2-C
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 62:13–25. https://doi.org/10.1016/j.freeradbiomed.2013.05.001
Li L, Shi L, Liu H, Luo Q, Huang C, Liu W, Chen X, Zeng W, Chen Z (2017) Changes in blood anti-oxidation enzyme levels in MPTP-treated monkeys. Neurosci Lett 10(649):93–99. https://doi.org/10.1016/j.neulet.2017.04.004
Watts RN, Hawkins C, Ponka P, Richardson DR (2006) Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc Natl Acad Sci USA 103:7670–7675. https://doi.org/10.1073/pnas.0602515103
Kunikowska G, Jenner P (2003) Alterations in m-RNA expression for Cu, Zn-superoxide dismutase and glutathione peroxidase in the basal ganglia of MPTP-treated marmosets and patients with Parkinson’s disease. Brain Res 2:206–218. https://doi.org/10.1016/s0006-8993(03)02240-6
Sutphin MS, Buckman TD (1991) Effects of low selenium diets on antioxidant status and MPTP toxicity in mice. Neurochem Res 12:1257–1263. https://doi.org/10.1007/BF00966655
Medda N, Patra R, Ghosh TK, Maiti S (2020) Neurotoxic mechanism of arsenic: synergistic effect of mitochondrial instability, oxidative stress, and hormonal-neurotransmitter impairment. Biol Trace Elem Res 198(1):8–15. https://doi.org/10.1007/s12011-020-02044-8
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 174(1):214–226. https://doi.org/10.1016/0003-2697(76)90326-2
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27(3):502–522. https://doi.org/10.1016/0003-2697(69)90064-5
Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C (2013) SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev 2013:836760. https://doi.org/10.1155/2013/836760
Kumar R, Nigam L, Singh AP, Singh K, Subbarao N, Dey S (2017) Design, synthesis of allosteric peptide activator for human SIRT and its biological evaluation in cellular model of Alzheimer’s disease. Eur J Med Chem 127:909–916. https://doi.org/10.1016/j.ejmech.2016.11.001
Cornelius C, Trovato Salinaro A, Scuto M, Fronte V, Cambria MT, Pennisi M, Bella R, Milone P, Graziano A, Crupi R et al (2013) Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: role of vitagenes. Immun Ageing 10(1):41. https://doi.org/10.1186/1742-4933-10-4
Peng S, Zhang B, Yao J, Duan D, Fang J (2015) Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PCcells. Food Funct 6(6):2091–2100. https://doi.org/10.1039/c5fo00097a
Calabrese V, Scapagnini G, Davinelli S, Koverech G, Koverech A, De Pasquale C, Salinaro AT, Scuto M, Calabrese EJ, Genazzani AR (2014) Sex hormonal regulation and hormesis in aging and longevity: role of vitagenes. J Cell Commun Signal 8(4):369–384. https://doi.org/10.1007/s12079-014-0253-7
Amara I, Timoumi R, Annabi E, Di Rosa G, Scuto M, Najjar MF, Calabrese V, Abid-Essefi S (2020) Di (2-ethylhexyl) phthalate targets the thioredoxin system and the oxidative branch of the pentose phosphate pathway in liver of Balb/c mice. Environ Toxicol 35(1):78–86. https://doi.org/10.1002/tox.22844
Perrone MA, Gualtieri P, Gratteri S, Ali W, Sergi D, Muscoli S, Cammarano A, Bernardini S, Di Rienzo L, Romeo E (2019) Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression: a nurtrigenomic approach for cardiovascular prevention. J Cardiovasc Med 20(7):419–426. https://doi.org/10.2459/JCM.0000000000000816
Oliveras-López MJ, Molina JJ, Mir MV, Rey EF, Martin F, de la Serrana HL (2013) Extra virgin olive oil (EVOO) consumption and antioxidant status in healthy institutionalized elderly humans. Arch Gerontol Geriatr 57(2):234–242. https://doi.org/10.1016/j.archger.2013.04.002
Pereira-Caro G, Madrona A, Bravo L, Espartero JL, Alcudia F, Cert A, Mateos R (2009) Antioxidant activity evaluation of alkyl hydroxytyrosyl ethers, a new class of hydroxytyrosol derivatives. Food Chem 115:86–91. https://doi.org/10.1016/j.foodchem.2008.11.069
Acknowledgements
This project was supported partially by CONACYT 840801. Pérez-Barrón is grateful to CONACYT (349836), to “Programa de Doctorado en Farmacia (UAEM) y CONACYT-MORELOS” (FOMIX 2013-1 # 224038) and to “Programa de Becas mixtas para becarios CONACYT nacionales: BECAS MIXTAS 2014—MZO2015 MOVILIDAD EN EL EXTRANJERO” (290842) for the scholarship Grants.
Funding
This project was supported partially by CONACYT and FORDECYT-PRONACES 840801. The funding agencies had no role in the design and conduct of the study; in analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
GP development the methodology, formal analysis, investigation and writing-original. AM and CR participated in conceptualization, formal analysis, investigation, resources, project administration, writing review and editing. SM, MS and JLE participated in supervision, conceptualization, resources, writing review and editing. YA and EG participated in methodology.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Ethical Approval
The experimental protocol was established, according with the ethical principles and regulations specified by the Animal Care and Use Committee of the National Institute of Neurology and Neurosurgery and the standards of the National Institutes of Health of Mexico.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pérez-Barrón, G., Montes, S., Aguirre-Vidal, Y. et al. Antioxidant Effect of Hydroxytyrosol, Hydroxytyrosol Acetate and Nitrohydroxytyrosol in a Rat MPP+ Model of Parkinson’s Disease. Neurochem Res 46, 2923–2935 (2021). https://doi.org/10.1007/s11064-021-03379-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03379-x