Abstract
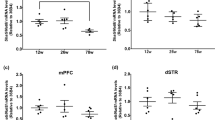
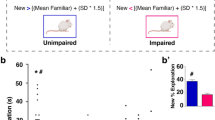
The sirtuin family of proteins consists of nicotinamide adenine dinucleotide-dependent deacetylases that are involved in the response to calorie restriction and various physiological phenomena, such as aging and cognition. One of these proteins, sirtuin 3 (SIRT3), is localized in the mitochondria and protects the cell against oxidative or metabolic stress. Sirtuin protein deficiencies have been shown to accelerate neurodegeneration in neurotoxic conditions. The mechanisms underlying the involvement of SIRT3 in cognition remain unclear. Interestingly, SIRT1, another member of the sirtuin family, has been reported to modulate synaptic plasticity and memory formation. To learn more about these proteins, we examined the behavior and cognitive functions of Sirt3-knockout mice. The mice exhibited poor remote memory. Consistent with this, long-term potentiation was impaired in the Sirt3-knockout mice, and they exhibited decreased neuronal number in the anterior cingulate cortex, which seemed to contribute to their memory deficiencies.




Similar content being viewed by others
References
Herskovits AZ, Guarente L (2013) Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23:746–758
Imai SI, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800
Rine J, Strathern JN, Hicks JB, Herskowitz I (1979) A suppressor of mating-type locus mutations in Saccharomyces cerevisiae: evidence for and identification of cryptic mating-type loci. Genetics 93:877–901
Nakagawa T, Guarente L (2011) Sirtuins at a glance. J Cell Sci 124:833–838
Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109
Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Guptaa MP (2014) SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34:807–819
Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP (2016) Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab 23:128–142
Magnifico S, Saias L, Deleglise B, Duplus E, Kilinc D, Miquel MC, Viovy JL, Brugg B, Peyrin JM (2013) NAD acts on mitochondrial SirT3 to prevent axonal caspase activation and axonal degeneration. FASEB J 27:4712–4722
Weir HJ, Murray TK, Kehoe PG, Love S, Verdin EM, O’Neill MJ, Lane JD, Balthasar N (2012) CNS SIRT3 expression is altered by reactive oxygen species and in Alzheimer’s disease. PLoS ONE 7: e48225
Yang W, Zou Y, Zhang M, Zhao N, Tian Q, Gu M, Liu W, Shi R, Lü Y, Yu W (2015) Mitochondrial Sirt3 expression is decreased in APP/PS1 double transgenic mouse model of Alzheimer’s disease. Neurochem Res 40:1576–1582
Gallart-Palau X, Lee BST, Adav SS, Qian J, Serra A, Park JE, Lai MKP, Chen CP, Kalaria RN, Sze SK (2016) Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer’s disease with cerebrovascular disease. Mol Brain 9(1): 27
Lillenes MS, Rabano A, Støen M, Riaz T, Misaghian D, Møllersen L, Esbensen Y, Günther CC, Selnes P, Stenset VTV, Fladby T, Tønjum T (2016) Altered DNA base excision repair profile in brain tissue and blood in Alzheimer’s disease. Mol Brain 9: 61
Kang SJ, Liu MG, Chen T, Ko HG, Baek GC, Lee HR, Lee K, Collingridge GL, Kaang BK, Zhuo M (2012) Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci 32:11318–11329
Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004) The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304:881–883
Wiltgen BJ, Brown RAM, Talton LE, Silva AJ (2004) New circuits for old memories: the role of the neocortex in consolidation. Neuron 44:101–108
Aceti M, Vetere G, Novembre G, Restivo L, Ammassari-Teule M (2015) Progression of activity and structural changes in the anterior cingulate cortex during remote memory formation. Neurobiol Learn Mem 123:67–71
Davies CA, Mann DMA, Sumpter PQ, Yates PO (1987) A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci 78:151–164
Scheff SW, Price DA, Schmitt FA, Dekosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68:1501–1508
Wang JF, Shao L, Sun X, Young LT (2009) Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord 11:523–529
Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–812
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352
Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N (2005) SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med 16:237–243
Li W, Zhang B, Tang J, Cao Q, Wu Y, Wu C, Guo J, Ling EA, Liang F (2007) Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating α-tubulin. J Neurosci 27:2606–2616
Jung ES, Choi H, Song H, Hwang YJ, Kim A, Ryu H, Mook-Jung I (2016) P53-dependent SIRT6 expression protects Aβ42-induced DNA damage. Sci Rep 6: 25628
Liu L, Peritore C, Ginsberg J, Kayhan M, Donmez G (2015) SIRT3 attenuates MPTP-induced nigrostriatal degeneration via enhancing mitochondrial antioxidant capacity. Neurochem Res 40:600–608
Acknowledgements
This work was supported by the National Honour Scientist Program of Korea (to B.K.K., NRF2012R1A3A1050385) and National Research Foundation of Korea (NRF) grants (to H.S.K., NRF2012R1A5A1048236 and NRF2012M3A9C5048708). We thank the members of the Kaang lab for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kim, H., Kim, S., Choi, J.E. et al. Decreased Neuron Number and Synaptic Plasticity in SIRT3-Knockout Mice with Poor Remote Memory. Neurochem Res 44, 676–682 (2019). https://doi.org/10.1007/s11064-017-2417-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2417-3