Abstract
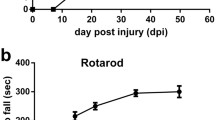
The incorporation of newborn neurons with increased synaptic remodeling and activity-dependent plasticity in the dentate gyrus enhances hippocampal-dependent learning performances. Astrocytes and microglial cells are components of the neurogenic niche and regulate neurogenesis under normal and neurophatological conditions leading to functional consequences for learning and memory. Although cognitive impairments were reported in patients after spinal cord injury (SCI), only few studies have considered remote changes in brain structures which are not related with sensory and motor cortex. Thus, we examined neurogenesis and glial reactivity by stereological assessment in dentate gyrus sub-regions after three different intensities of thoracic spinal cord compression in rats. Sixty days after injury we observed a decrease in the Basso–Bresnahan–Beattie locomotor scale scores, rotarod performance and volume of spare tissue that correlated with the severity of the compression. Regarding the hippocampus, we observed that neurogenesis and hilar neurons were reduced after severe SCI, while only neurogenesis decreased in the moderately injured group. In addition, severe SCI induced reactive microglia and astrogliosis in all dentate gyrus sub-regions. Furthermore, the density of reactive microglia increased in the hilus whereas astrogliosis developed in the molecular layer after moderate SCI. No changes were observed in the mildly injured rats. These results suggest glial response and neurogenesis are associated with injury intensity. Interestingly, hippocampal neurogenesis is more sensitive to SCI than astrocytes or microglia reaction, as moderate injury impairs the generation of new neurons without changing glial response in the subgranular zone.





Similar content being viewed by others
References
O´Keefe J (2007) Hippocampal neurophysiology in the behaving animal. In: Andersen PMR, Amaral D, Bliss T, O´Keefe J (eds) The hippocampus book. Oxford University press, Oxford, p 475–548
Deng W, Saxe MD, Gallina IS, Gage FH (2009) Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci 29(43):13532–13542. doi:10.1523/JNEUROSCI.3362-09.2009
Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW (2011) Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci 31(42):15113–15127. doi:10.1523/JNEUROSCI.3432-11.2011
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. doi:10.1016/j.neuron.2011.05.001
Gage FH (2000) Mammalian neural stem cells. Science 287(5457):1433–1438
Kempermann G, Gage FH (1999) Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus 9(3):321–332. doi:10.1002/(SICI)1098-1063(1999)9:3<321::AID-HIPO11>3.0.CO;2-C
Ehninger D, Kempermann G (2008) Neurogenesis in the adult hippocampus. Cell Tissue Res 331(1):243–250. doi:10.1007/s00441-007-0478-3
Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, Steinhauser C, Gray WP (2003) Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci 18(10):2769–2778
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell stem cell 7(4):483–495. doi:10.1016/j.stem.2010.08.014
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415(6875):1030–1034. doi:10.1038/4151030a
Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L (2005) Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci 21(1):1–14. doi:10.1111/j.1460-9568.2004.03813.x
Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF (2008) Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11(8):901–907. doi:10.1038/nn.2156
Lin R, Iacovitti L (2015) Classic and novel stem cell niches in brain homeostasis and repair. Brain research 1628(Pt B):327–342. doi:10.1016/j.brainres.2015.04.029
Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME (2014) Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast 2014:610343. doi:10.1155/2014/610343
Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW, Sun LR, Xiong WC, Gao TM, Zhu XH (2013) Astrocytic adenosine 5′-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem cells 31(8):1633–1643. doi:10.1002/stem.1408
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417(6884):39–44. doi:10.1038/417039a
Sultan S, Li L, Moss J, Petrelli F, Casse F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, Toni N (2015) Synaptic integration of adult-born hippocampal neurons is locally Controlled by astrocytes. Neuron 88(5):957–972. doi:10.1016/j.neuron.2015.10.037
Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci 31(45):16064–16069. doi:10.1523/JNEUROSCI.4158-11.2011
Davidoff GN, Roth EJ, Richards JS (1992) Cognitive deficits in spinal cord injury: epidemiology and outcome. Arch Phys Med Rehabil 73(3):275–284
Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K (1997) Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc 3(5):464–472
Lazzaro I, Tran Y, Wijesuriya N, Craig A (2013) Central correlates of impaired information processing in people with spinal cord injury. J Clin Neurophysiol 30(1):59–65. doi:10.1097/WNP.0b013e31827edb0c
Wu J, Stoica BA, Luo T, Sabirzhanov B, Zhao Z, Guanciale K, Nayar SK, Foss CA, Pomper MG, Faden AI (2014) Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation. Cell Cycle 13(15):2446–2458. doi:10.4161/cc.29420
Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI (2014) Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci34(33):10989–11006. doi:10.1523/JNEUROSCI.5110-13.2014
Fehlings MG, Tator CH (1995) The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol 132(2):220–228
Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, Rossignol S (2011) Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J Neurotrauma 28(9):1963–1981. doi:10.1089/neu.2011.1840
Moonen G, Satkunendrarajah K, Wilcox JT, Badner A, Mothe A, Foltz W, Fehlings MG, Tator CH (2016) A new acute impact-compression lumbar spinal cord injury model in the rodent. J Neurotrauma 33(3):278–289. doi:10.1089/neu.2015.3937
Dolan EJ, Tator CH (1979) A new method for testing the force of clips for aneurysms or experimental spinal cord compression. J Neurosurg 51(2):229–233. doi:10.3171/jns.1979.51.2.0229
Khan M, Griebel R (1983) Acute spinal cord injury in the rat: comparison of three experimental techniques. Can J Neurol Sci Le J Can Des Sci Neurol 10(3):161–165
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139(2):244–256
Garcia-Ovejero D, Gonzalez S, Paniagua-Torija B, Lima A, Molina-Holgado E, De Nicola AF, Labombarda F (2014) Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. J Neurotrauma 31(9):857–871. doi:10.1089/neu.2013.3162
Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr (2003) Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20(2):179–193. doi:10.1089/08977150360547099
Adamczyk A, Mejias R, Takamiya K, Yocum J, Krasnova IN, Calderon J, Cadet JL, Huganir RL, Pletnikov MV, Wang T (2012) GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. Behav Brain Res 229(1):265–272. doi:10.1016/j.bbr.2012.01.007
Paxinos GaW C (2007) The rat brain in the stereotaxic coordinates. Elsevier, London
Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF (2015) A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2015.09.011
Pietranera L, Saravia F, Gonzalez Deniselle MC, Roig P, Lima A, De Nicola AF (2006) Abnormalities of the hippocampus are similar in deoxycorticosterone acetate-salt hypertensive rats and spontaneously hypertensive rats. J Neuroendocrinol 18(6):466–474. doi:10.1111/j.1365-2826.2006.01436.x
Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130(4):813–831
Soltys Z, Ziaja M, Pawlinski R, Setkowicz Z, Janeczko K (2001) Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res 63(1):90–97
Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A (2004) Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478(4):359–378. doi:10.1002/cne.20288
Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96 (10):857–881
Ekdahl CT (2012) Microglial activation—tuning and pruning adult neurogenesis. Front Pharmacol 3:41. doi:10.3389/fphar.2012.00041
Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G, Giros B, Mechawar N (2014) Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation 11:12. doi:10.1186/1742-2094-11-12
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647. doi:10.1016/j.tins.2009.08.002
Felix MS, Popa N, Djelloul M, Boucraut J, Gauthier P, Bauer S, Matarazzo VA (2012) Alteration of forebrain neurogenesis after cervical spinal cord injury in the adult rat. Front Neurosci 6:45. doi:10.3389/fnins.2012.00045
Azcoitia I, Sierra A, Garcia-Segura LM (1998) Estradiol prevents kainic acid-induced neuronal loss in the rat dentate gyrus. Neuroreport 9(13):3075–3079
Saravia F, Beauquis J, Pietranera L, De Nicola AF (2007) Neuroprotective effects of estradiol in hippocampal neurons and glia of middle age mice. Psychoneuroendocrinology 32(5):480–492. doi:10.1016/j.psyneuen.2007.02.012
Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A (2009) Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA 106(35):14978–14983. doi:10.1073/pnas.0809784106
Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O (2006) Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci 26(38):9703–9712. doi:10.1523/JNEUROSCI.2723-06.2006
Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T, Narita M (2010) Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse 64(9):721–728. doi:10.1002/syn.20800
Vallieres L, Campbell IL, Gage FH, Sawchenko PE (2002) Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 22(2):486–492
Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T (2010) CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP + PS1 bigenic mice. FASEB J 24 (8):3093–3102. doi:10.1096/fj.10-155317
Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T (2012) AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP + PS1 mice. Gene Ther 19(7):724–733. doi:10.1038/gt.2011.126
Mathieu P, Piantanida AP, Pitossi F (2010) Chronic expression of transforming growth factor-beta enhances adult neurogenesis. Neuroimmunomodulation 17(3):200–201. doi:10.1159/000258723
Lu Z, Kipnis J (2010) Thrombospondin 1−a key astrocyte-derived neurogenic factor. FASEB J 24 (6):1925–1934. doi:10.1096/fj.09-150573
Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS (2009) Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology 34(3):747–758. doi:10.1038/npp.2008.136
Saravia FE, Beauquis J, Revsin Y, Homo-Delarche F, de Kloet ER, De Nicola AF (2006) Hippocampal neuropathology of diabetes mellitus is relieved by estrogen treatment. Cell Mol Neurobiol 26(4–6):943–957. doi:10.1007/s10571-006-9096-y
Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell stem cell 8(5):566–579. doi:10.1016/j.stem.2011.03.010
Sierra A, Martin-Suarez S, Valcarcel-Martin R, Pascual-Brazo J, Aelvoet SA, Abiega O, Deudero JJ, Brewster AL, Bernales I, Anderson AE, Baekelandt V, Maletic-Savatic M, Encinas JM (2015) Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell stem cell 16(5):488–503. doi:10.1016/j.stem.2015.04.003
Lee J, Duan W, Mattson MP (2002) Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82(6):1367–1375
Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192(2):348–356. doi:10.1016/j.expneurol.2004.11.016
Fumagalli F, Madaschi L, Caffino L, Marfia G, Di Giulio AM, Racagni G, Gorio A (2009) Acute spinal cord injury reduces brain derived neurotrohic factor expression in rat hippocampus. Neuroscience 159(3):936–939. doi:10.1016/j.neuroscience.2009.01.030
Nardone R, Holler Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S, Trinka E (2013) Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res 1504:58–73. doi:10.1016/j.brainres.2012.12.034
Amaral DaL P (2007) Hippocampal neuroanatomy. In: Andersen P MR, Amaral D, Bliss T, O´Keefe J (eds) The hippocampus BOOK. Oxford University press, Oxford pp 37–114
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924. doi:10.1038/nn1715
Gundersen V, Storm-Mathisen J, Bergersen LH (2015) Neuroglial transmission. Physiol Rev 95(3):695–726. doi:10.1152/physrev.00024.2014
Zhao P, Waxman SG, Hains BC (2007) Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci 27(33):8893–8902. doi:10.1523/JNEUROSCI.2209-07.2007
Popovich P, McTigue D (2009) Damage control in the nervous system: beware the immune system in spinal cord injury. Nat Med 15(7):736–737. doi:10.1038/nm0709-736
Schwab JM, Zhang Y, Kopp MA, Brommer B, Popovich PG (2014) The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp Neurol 258:121–129. doi:10.1016/j.expneurol.2014.04.023
Lucassen PJ, Oomen CA, Naninck EF, Fitzsimons CP, van Dam AM, Czeh B, Korosi A (2015) Regulation of adult neurogenesis and plasticity by (Early) stress, glucocorticoids, and inflammation. Cold Spring Harbor Perspect Biol 7 (9):a021303.doi:10.1101/cshperspect.a021303
Acknowledgements
We are grateful to Dr Juan Manuel Encinas for his helpful comments and generosity in revising this manuscript.
Funding
This work was supported by grants from the Ministry of Science and Technology (PICT 2012-0009), the National Research Council of Argentina (PIP 112 20120100016), the University of Buenos Aires (Ubacyt 20020100100089) and Roemmers Fundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Jure, I., Pietranera, L., De Nicola, A.F. et al. Spinal Cord Injury Impairs Neurogenesis and Induces Glial Reactivity in the Hippocampus. Neurochem Res 42, 2178–2190 (2017). https://doi.org/10.1007/s11064-017-2225-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2225-9