Abstract
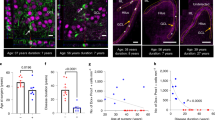
The HMGB1-TLR4 axis is activated in adult mouse models of acute and chronic seizure. Nevertheless, whether HMGB1 was involved in the pathogenesis of mesial temporal lobe epilepsy (MTLE) remains unknown. In this study, we first measured the dynamic expression patterns of HMGB1 and TLR4 in the hippocampi of a rat model and in children with MTLE, as well as the levels of TNF-α and IL-1β. In addition, HMGB1 was added to mimic the process of inflammatory response in neurons. Neuronal somatic size and dendritic length were measured by immunohistochemistry and digital imaging. The results showed that the expression of HMGB1 and TLR4 as well as the levels of TNF-α and IL-1β were higher in the three stages of MTLE development in the rat model and in the children with MTLE. HMGB1 increased the levels of TNF-α and IL-1β, upregulated the protein level of p-p38MAPK and promoted the growth of cell somatic size and dendritic length in neurons. Pre-treatment with p38MAPK inhibitor SB203580 decreased the levels of TNF-α and IL-1β, while downregulation of TLR4 significantly reduced HMGB1-induced p38MAPK signaling pathway activation. These data demonstrated that the HMGB1-TLR4 axis may play an important role in the pathogenesis of MTLE via the p38MAPK signaling pathway.







Similar content being viewed by others
References
Abubakar A, Kariuki SM, Tumaini JD, Gona J, Katana K, Owen JA, Newton CR (2015) Community perceptions of developmental and behavioral problems experienced by children living with epilepsy on the Kenyan coast: a qualitative study. Epilepsy Behav 45:74–78
Palleria C, Coppola A, Citraro R, Del Gaudio L, Striano S, De Sarro G, Russo E (2015) Perspectives on treatment options for mesial temporal lobe epilepsy with hippocampal sclerosis. Expert Opin Pharmacother 16:2355–2371
Zhang Y, Li Z, Gu J, Wang W, Shen H, Chen G, Wang X (2015) Plic-1, a new target in repressing epileptic seizure by regulation of GABAAR function in patients and a rat model of epilepsy. Clin Sci (Lond) 129:1207–1223
Toller G, Adhimoolam B, Rankin KP, Huppertz HJ, Kurthen M, Jokeit H (2015) Right fronto-limbic atrophy is associated with reduced empathy in refractory unilateral mesial temporal lobe epilepsy. Neuropsychologia 78:80–87
Sillanpaa M, Anttinen A, Rinne JO, Joutsa J, Sonninen P, Erkinjuntti M, Hermann B, Karrasch M, Saarinen M, Tiitta P, Shinnar S (2015) Childhood-onset epilepsy five decades later. A prospective population-based cohort study. Epilepsia 56:1774–1783
Busch RM, Lineweaver TT, Ferguson L, Haut JS (2015) Reliable change indices and standardized regression-based change score norms for evaluating neuropsychological change in children with epilepsy. Epilepsy Behav 47:45–54
Luoni C, Canevini MP, Capovilla G, De Sarro G, Galimberti CA, Gatti G, Guerrini R, La Neve A, Mazzucchelli I, Rosati E, Specchio LM, Striano S, Tinuper P, Perucca E (2015) A prospective study of direct medical costs in a large cohort of consecutively enrolled patients with refractory epilepsy in Italy. Epilepsia 56:1162–1173
Brennan GP, Dey D, Chen Y, Patterson KP, Magnetta EJ, Hall AM, Dube CM, Mei YT, Baram TZ (2016) Dual and opposing roles of microRNA-124 in epilepsy are mediated through inflammatory and NRSF-dependent gene networks. Cell Rep 14:2402–2412
Hamid KM, Nejati A, Shoja Z, Mollaei-Kandelousd Y, Doosti R, Mirshafiey A, Tafakhori A, Sahraian MA, Marashi SM (2016) Quantitative evaluation of BAFF, HMGB1, TLR 4 AND TLR 7 expression in patients with relapsing remitting multiple sclerosis. Iran J Allergy Asthma Immunol 15:75–81
Su S, Wu J, Gong T, He K, Feng C, Zhang M, Li B, Xia X (2016) Inhibition of high mobility group box 1-toll-like receptor-4 signaling by glycyrrhizin contributes to the attenuation of cold ischemic injury of liver in a rat model. Transplant Proc 48:191–198
Li G, Wu X, Yang L, He Y, Liu Y, Jin X, Yuan H (2016) TLR4-mediated NF-kappaB signaling pathway mediates HMGB1-induced pancreatic injury in mice with severe acute pancreatitis. Int J Mol Med 37:99–107
Lee K, Chang Y, Song K, Park YY, Huh JW, Hong SB, Lim CM, Koh Y (2016) Associations between single nucleotide polymorphisms of high mobility group box 1 protein and clinical outcomes in korean sepsis patients. Yonsei Med J 57:111–117
Wang WJ, Yin SJ, Rong RQ (2015) PKR and HMGB1 expression and function in rheumatoid arthritis. Genet Mol Res 14:17864–17870
Ma L, Zeng J, Mo B, Wang C, Huang J, Sun Y, Yu Y, Liu S (2015) High mobility group box 1: a novel mediator of Th2-type response-induced airway inflammation of acute allergic asthma. J Thorac Dis 7:1732–1741
Jiang WL, Xu Y, Zhang SP, Zhu HB, Hou J (2012) Tricin 7-glucoside protects against experimental cerebral ischemia by reduction of NF-kappaB and HMGB1 expression. Eur J Pharm Sci 45:50–57
Okuma Y, Date I, Nishibori M (2014) Anti-HMGB1 antibody therapy for traumatic brain injury and neuropathic pain. Nihon Yakurigaku Zasshi 143:5–9
Miyasho T, Nakamura K, Nomura S, Kawasako K, Nakade T, Yamada S, Yokota H (2011) High mobility group box 1 (HMGB1) protein is present in the cerebrospinal fluid of dogs with encephalitis. J Vet Med Sci 73:917–922
Kim J, Song J, Lee M (2015) Combinational delivery of HMGB1 A box and heparin for acute lung injury. J Control Release 213:e57
Ye L, Yang Y, Zhang X, Cai P, Li R, Chen D, Wei X, Xu H, Xiao J, Li X, Lin L, Zhang H (2016) The role of bFGF in the excessive activation of astrocytes is related to the inhibition of TLR4/NFkappaB signals. Int J Mol Sci 17:37
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–419
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Kaech S, Banker G (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415
Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, Agresti A, Antonelli A, Bianchi ME, Aronica E, Ravizza T, Vezzani A (2013) Receptor for advanced glycation endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol Dis 58:102–114
Sowell MK, Youssef PE (2016) The comorbidity of migraine and epilepsy in children and adolescents. Semin Pediatr Neurol 23:83–91
Nascimento FA, Gatto LA, Silvado C, Mader-Joaquim MJ, Moro MS, Araujo JC (2016) Anterior temporal lobectomy versus selective amygdalohippocampectomy in patients with mesial temporal lobe epilepsy. Arq Neuropsiquiatr 74:35–43
Marchi N, Granata T, Janigro D (2014) Inflammatory pathways of seizure disorders. Trends Neurosci 37:55–65
Dube CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30:7484–7494
Li W, Wang X, Niu X, Zhang H, He Z, Wang Y, Zhi W, Liu F (2016) Protective effects of nobiletin against endotoxic shock in mice through inhibiting TNF-alpha, IL-6, and HMGB1 and regulating NF-kappaB pathway. Inflammation 39:786–797
Taniguchi N, Kawahara K, Yone K, Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K, Matsunaga S, Nakajima T, Komiya S, Maruyama I (2003) High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum 48:971–981
Ombrellino M, Wang H, Ajemian MS, Talhouk A, Scher LA, Friedman SG, Tracey KJ (1999) Increased serum concentrations of high-mobility-group protein 1 in haemorrhagic shock. Lancet 354:1446–1447
Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld AN, Wang H, Lesser ML, Church AL, Litroff AH, Sama AE, Tracey KJ (2006) Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock 25:571–574
Ueno H, Matsuda T, Hashimoto S, Amaya F, Kitamura Y, Tanaka M, Kobayashi A, Maruyama I, Yamada S, Hasegawa N, Soejima J, Koh H, Ishizaka A (2004) Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 170:1310–1316
Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y (2006) Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas 33:359–363
Zare-Shahabadi A, Ashrafi MR, Shahrokhi A, Soltani S, Zoghi S, Soleimani F, Vameghi R, Badv RS, Rezaei N (2015) Single nucleotide polymorphisms of TNF-Alpha gene in febrile seizures. J Neurol Sci 356:153–156
Akassoglou K, Probert L, Kontogeorgos G, Kollias G (1997) Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J Immunol 158:438–445
Diamond ML, Ritter AC, Failla MD, Boles JA, Conley YP, Kochanek PM, Wagner AK (2015) IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia 56:991–1001
Rotenberg A (2015) Commentary on IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia 56:989–990
Xiao Z, Peng J, Yang L, Kong H, Yin F (2015) Interleukin-1beta plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J Neuroimmunol 282:110–117
Acknowledgements
There is no funds to support this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to declare.
Additional information
Weihong Yang and Jing Li have contributed equally to the study.
Rights and permissions
About this article
Cite this article
Yang, W., Li, J., Shang, Y. et al. HMGB1-TLR4 Axis Plays a Regulatory Role in the Pathogenesis of Mesial Temporal Lobe Epilepsy in Immature Rat Model and Children via the p38MAPK Signaling Pathway. Neurochem Res 42, 1179–1190 (2017). https://doi.org/10.1007/s11064-016-2153-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2153-0