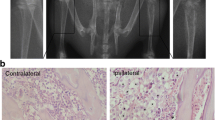
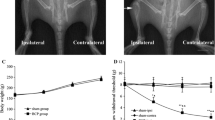
Abstract
Cancer-induced bone pain (CIBP) is a challenging medical problem that considerably influences cancer patients’ quality of life. Currently, few treatments have been developed to conquer CIBP because of a poor understanding of the potential mechanisms. Our previous work has proved that spinal RANTES (a major ligand for CCR5) was involved in the maintenance of CIBP. In this study, we attempted to investigate whether spinal CCR5 and its downstream PKCγ pathway is involved in the maintenance of CIBP. Inoculation of Walker 256 cells into the tibia could induce a marked mechanical allodynia with concomitant upregulation of spinal CCR5 and p-PKCγ expression from day 6 to day 15 after inoculation. Spinal CCR5 was prominently expressed in microglia, and mechanical allodynia was attenuated by intrathecal injection of DAPTA (a specific antagonist of CCR5) with downregulation of spinal CCR5 and p-PKCγ expression levels at day 15 in inoculated rats. Pre-intrathecal injection of RANTES could reverse the anti-allodynia effects of DAPTA. Intrathecal administration of GF109203X (an inhibitor of PKC) could alleviate mechanical allodynia as well as decrease of spinal p-PKCγ expression level, but no influence on spinal CCR5 level. Our findings suggest that CCR5/PKCγ signaling pathway in microglia may contribute to the maintenance of CIBP in rats.




Similar content being viewed by others
References
van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA et al (2016) Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage 51(6):1070–1090
Hendriks LE, Hermans BC, van den Beuken-van Everdingen MH et al (2016) Effect of bisphosphonates, denosumab, and radioisotopes on bone pain and quality of life in patients with non-small cell lung cancer and bone metastases: a systematic review. J Thorac Oncol 11(2):155–173
Reis-Pina P, Lawlor PG, Barbosa A (2015) Cancer-related pain management and the optimal use of opioids. Acta Med Port 28(3):376–381
von Moos R, Body JJ, Egerdie B et al (2016) Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer 24(3):1327–1337
Patrick DL, Cleeland CS, von Moos R et al (2015) Pain outcomes in patients with bone metastases from advanced cancer: assessment and management with bone-targeting agents. Support Care Cancer 23(4):1157–1168
Schug SA, Chandrasena C (2015) Pain management of the cancer patient. Expert Opin Pharmacother 16(1):5–15
Muralidharan A, Smith MT (2013) Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology 21(5):339–363
Llorián-Salvador M, González- Rodríguez S, Lastra A (2016) Involvement of CC Chemokine Receptor 1 and CCL3 in Acute and Chronic Inflammatory Pain in Mice. Basic Clin Pharmacol Toxicol 119(1):32–40
Guo G, Gao F (2015) CXCR3: latest evidence for the involvement of chemokine signaling in bone cancer pain. Exp Neurol 265:176–179
Hang LH, Li SN, Shao DH et al (2014) Evidence for involvement of spinal RANTES in the antinociceptive effects of triptolide, a diterpene triepoxide, in a rat model of bone cancer pain. Basic Clin Pharmacol Toxicol 115(6):477–480
Tang J, Li ZH, Ge SN et al (2012) The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med 2012:185167.
Xu F, Li Y, Li S et al (2014) Complete Freund’s adjuvant-induced acute inflammatory pain could be attenuated by triptolide via inhibiting spinal glia activation in rats. J Surg Res 188(1):174–182
Hang LH, Shao DH, Chen Z et al (2013) Involvement of spinal cc chemokine ligand 5 in the development of bone cancer pain in rats. Basic Clin Pharmacol Toxicol 113(5):325–328
Wu Y, Yoder A (2009) Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog 5(12):e1000520
Koda K, Hyakkoku K, Ogawa K et al (2016) Sensitization of TRPV1 by protein kinase C in rats with mono-iodoacetate-induced joint pain. Osteoarthritis Cartilage 24(7):1254–1262
de Souza Nunes JP, da Silva KA, da Silva GF et al (2014) The antihypersensitive and antiinflammatory activities of a benzofuranone derivative in different experimental models in mice: the importance of the protein kinase C pathway. Anesth Analg 119(4):836–846
Loram LC, Taylor FR, Strand KA et al (2013) Intrathecal injection of adenosine 2 A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav Immun 33:112–122
Hang LH, Yang JP, Yin W et al (2012) Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur J Neurosci 36(1):2107–2117
Hang LH, Li SN, Luo H et al (2016) Connexin 43 mediates CXCL12 production from spinal dorsal horn to maintain bone cancer pain in rats. Neurochem Res 41(5):1200–1208
Guan XH, Fu QC, Shi D et al (2015) Activation of spinal chemokine receptor CXCR3 mediates bone cancer pain through an Akt-ERK crosstalk pathway in rats. Exp Neurol 263:39–49
Hang LH, Luo H, Li SN et al (2015) Involvement of spinal Bv8/Prokineticin 2 in a rat model of cancer-induced bone pain. Basic Clin Pharmacol Toxicol 117(3):180–185
Zhou YQ, Gao HY, Guan XH et al (2015) Chemokines and their receptors: potential therapeutic targets for bone cancer pain. Curr Pharm Des 21(34):5029–5033
Alkhatib G (2009) The biology of CCR5 and CXCR4. Curr Opin HIV AIDS 4(2):96–103
Matsushita K, Tozaki-Saitoh H, Kojima C et al (2014) Chemokine (C-C motif) receptor 5 is an important pathological regulator in the development and maintenance of neuropathic pain. Anesthesiology 120(6):1491–1503
Sun S, Chen D, Lin F et al (2016) Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol Immunol 77:184–192
Saika F, Kiguchi N, Kobayashi Y et al (2012) CC-chemokine ligand 4/macrophage inflammatory protein-1β participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 16(9):1271–1280
Lee YK, Choi DY, Jung YY et al (2013) Decreased pain responses of C-C chemokine receptor 5 knockout mice to chemical or inflammatory stimuli. Neuropharmacology 67:57–65
Liou JT, Mao CC, Ching-Wah Sum D et al (2013) Peritoneal administration of Met-RANTES attenuates inflammatory and nociceptive responses in a murine neuropathic pain model. J Pain 14 (1): 24–35.
Padi SS, Shi XQ, Zhao YQ et al (2012) Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain 153(1):95–106
Páldy E, Bereczki E, Sántha M et al (2008) CB(2) cannabinoid receptor antagonist SR144528 decreases mu-opioid receptor expression and activation in mouse brainstem: role of CB(2) receptor in pain. Neurochem Int 53(6–8):309–316
Hüttenrauch F, Pollok-Kopp B, Oppermann M (2005) G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem 280(45):37503–37515
Mori M, Kose A, Tsujino T et al (1990) Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol 299(2):167–177
Malmberg AB, Chen C, Tonegawa S, Basbaum AI (1997) Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 278(5336):279–283
Bu F, Tian H, Gong S et al (2015) Phosphorylation of NR2B NMDA subunits by protein kinase C in arcuate nucleus contributes to inflammatory pain in rats. Sci Rep 5:15945
Honore P, Rogers SD, Schwei MJ et al (2000) Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98(3):585–598
Schwei MJ, Honore P, Rogers SD et al (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 19(24):10886–10897
Zhang MY, Liu YP, Zhang LY et al (2015) Levo-tetrahydropalmatine attenuates bone cancer pain by inhibiting microglial cells activation. Mediators Inflamm 2015:752512
Yang Y, Li H, Li TT et al (2015) Delayed activation of spinal microglia contributes to the maintenance of bone cancer pain in female Wistar rats via P2 × 7 receptor and IL-18. J Neurosci 35(20):7950–7963
Wang LN, Yang JP, Zhan Y et al (2012) Minocycline-induced reduction of brain-derived neurotrophic factor expression in relation to cancer-induced bone pain in rats. J Neurosci Res 90(3):672–681
Hu JH, Yang JP, Liu L et al (2012) Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res 1465:1–9
Liu S, Yang J, Wang L et al (2010) Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res 1346:213–223
Liou JT, Lee CM, Day YJ (2013) The immune aspect in neuropathic pain: role of chemokines. Acta Anaesthesiol Taiwan 51(3):127–132
Acknowledgements
This work was financed by the Social Development Fund of Zhenjiang, Jiangsu Province (SH2015049) and the Natural Science Foundation of the PR China (No. 81600799).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hang, LH., Li, SN., Dan, X. et al. Involvement of Spinal CCR5/PKCγ Signaling Pathway in the Maintenance of Cancer-Induced Bone Pain. Neurochem Res 42, 563–571 (2017). https://doi.org/10.1007/s11064-016-2108-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2108-5