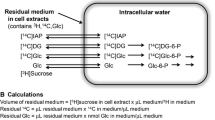
Abstract
The 13C turnover of neurotransmitter amino acids (glutamate, GABA and aspartate) were determined from extracts of forebrain nerve terminals and brain homogenate, and fronto-parietal cortex from anesthetized rats undergoing timed infusions of [1,6-13C2]glucose or [2-13C]acetate. Nerve terminal 13C fractional labeling of glutamate and aspartate was lower than those in whole cortical tissue at all times measured (up to 120 min), suggesting either the presence of a constant dilution flux from an unlabeled substrate or an unlabeled (effectively non-communicating on the measurement timescale) glutamate pool in the nerve terminals. Half times of 13C labeling from [1,6-13C2]glucose, as estimated by least squares exponential fitting to the time course data, were longer for nerve terminals (GluC4, 21.8 min; GABAC2 21.0 min) compared to cortical tissue (GluC4, 12.4 min; GABAC2, 14.5 min), except for AspC3, which was similar (26.5 vs. 27.0 min). The slower turnover of glutamate in the nerve terminals (but not GABA) compared to the cortex may reflect selective effects of anesthesia on activity-dependent glucose use, which might be more pronounced in the terminals. The 13C labeling ratio for glutamate-C4 from [2-13C]acetate over that of 13C-glucose was twice as large in nerve terminals compared to cortex, suggesting that astroglial glutamine under the 13C glucose infusion was the likely source of much of the nerve terminal dilution. The net replenishment of most of the nerve terminal amino acid pools occurs directly via trafficking of astroglial glutamine.




Similar content being viewed by others
References
van den Berg CJ, Garfinkel D (1971) A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J 123:211–218
van den Berg CJ, Krzalic L, Mela P, Waelsch H (1969) Compartmentation of glutamate metabolism in brain. Evidence for the existence of two different tricarboxylic acid cycles in brain. Biochem J 113:281–290
Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U (2015) The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res 40:402–409
Schousboe A (2003) Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem Res 28:347–352
Erecinska M, Zaleska MM, Nissim I, Nelson D, Dagani F, Yudkoff M (1988) Glucose and synaptosomal glutamate metabolism: studies with [15N]glutamate. J Neurochem 51:892–902
McKenna MC, Tildon JT, Stevenson JH, Hopkins IB (1994) Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: evidence for multiple compartments of tricarboxylic acid cycle activity. Dev Neurosci 16:291–300
Yudkoff M, Zaleska MM, Nissim I, Nelson D, Erecinska M (1989) Neuronal glutamine utilization: pathways of nitrogen transfer studied with [15N]glutamine. J Neurochem 53:632–640
McKenna MC (2013) Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 4:191
McKenna MC (2007) The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res 85:3347–3358
Hertz L, Yu AC, Kala G, Schousboe A (2000) Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem Int 37:83–102
Sonnewald U (2014) Glutamate synthesis has to be matched by its degradation—where do all the carbons go? J Neurochem 131:399–406
Badar-Goffer RS, Bachelard HS, Morris PG (1990) Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J 266:133–139
Sonnewald U, Westergaard N, Jones P, Taylor A, Bachelard HS, Schousboe A (1996) Metabolism of [U-13C5] glutamine in cultured astrocytes studied by NMR spectroscopy: first evidence of astrocytic pyruvate recycling. J Neurochem 67:2566–2572
Sonnewald U, Westergaard N, Schousboe A, Svendsen JS, Unsgard G, Petersen SB (1993) Direct demonstration by 13C NMR spectroscopy that glutamine from astrocytes is a precursor for GABA synthesis in neurons. Neurochem Int 22:19–29
Kanamori K, Kondrat RW, Ross BD (2003) 13C enrichment of extracellular neurotransmitter glutamate in rat brain–combined mass spectrometry and NMR studies of neurotransmitter turnover and uptake into glia in vivo. Cell Mol Biol (Noisy-le-grand) 49:819–836
Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (1999) Synthesis of vesicular GABA from glutamine involves TCA cycle metabolism in neocortical neurons. J Neurosci Res 57:342–349
Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (2001) Multiple compartments with different metabolic characteristics are involved in biosynthesis of intracellular and released glutamine and citrate in astrocytes. Glia 35:246–252
Waagepetersen HS, Sonnewald U, Schousboe A (2003) Compartmentation of glutamine, glutamate, and GABA metabolism in neurons and astrocytes: functional implications. Neuroscientist 9:398–403
Fitzpatrick SM, Hetherington HP, Behar KL, Shulman RG (1990) The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed 13C-edited NMR spectroscopy. J Cereb Blood Flow Metab 10:170–179
Cerdan S, Kunnecke B, Seelig J (1990) Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem 265:12916–12926
Shen J, Sibson NR, Cline G, Behar KL, Rothman DL, Shulman RG (1998) 15N-NMR spectroscopy studies of ammonia transport and glutamine synthesis in the hyperammonemic rat brain. Dev Neurosci 20:434–443
Gruetter R, Novotny EJ, Boulware SD, Mason GF, Rothman DL, Shulman GI, Prichard JW, Shulman RG (1994) Localized 13C NMR spectroscopy in the human brain of amino acid labeling from D-[1-13C]glucose. J Neurochem 63:1377–1385
Gruetter R, Seaquist ER, Ugurbil K (2001) A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab 281:E100–E112
Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL (2005) The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci USA 102:5588–5593
Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R (2004) Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci 24:11273–11279
Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG (1997) In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA 94:2699–2704
Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA 95:316–321
Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL (1999) Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci USA 96:8235–8240
Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL (2011) 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed 24:943–957
Bluml S, Moreno A, Hwang JH, Ross BD (2001) [1-13C] glucose magnetic resonance spectroscopy of pediatric and adult brain disorders. NMR Biomed 14:19–32
Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ (1995) Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J Cereb Blood Flow Metab 15:12–25
Mason GF, Rothman DL (2004) Basic principles of metabolic modeling of NMR 13C isotopic turnover to determine rates of brain metabolism in vivo. Metabol Eng 6:75–84
Mason GF, Rothman DL, Behar KL, Shulman RG (1992) NMR determination of the TCA cycle rate and alpha-ketoglutarate/glutamate exchange rate in rat brain. J Cereb Blood Flow Metab 12:434–447
Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG (2001) In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem 76:975–989
Duarte JM, Gruetter R (2013) Glutamatergic and GABAergic energy metabolism measured in the rat brain by 13C NMR spectroscopy at 14.1T. J Neurochem 126:579–590
Choi IY, Lei H, Gruetter R (2002) Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab 22:1343–1351
Henry PG, Lebon V, Vaufrey F, Brouillet E, Hantraye P, Bloch G (2002) Decreased TCA cycle rate in the rat brain after acute 3-NP treatment measured by in vivo 1H-[13C]-NMR spectroscopy. J Neurochem 82:857–866
de Graaf RA, Mason GF, Patel AB, Rothman DL, Behar KL (2004) Regional glucose metabolism and glutamatergic neurotransmission in rat brain in vivo. Proc Natl Acad Sci USA 101:12700–12705
Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, Shulman RG, Behar KL (2004) Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J Cereb Blood Flow Metab 24:972–985
Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL (2002) Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22:1523–1531
Hyder F, Fulbright RK, Shulman RG, Rothman DL (2013) Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J Cereb Blood Flow Metab 33:339–347
Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21:1133–1145
Hyder F, Rothman DL, Bennett MR (2013) Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci USA 110:3549–3554
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999) Energy on demand. Science 283:496–497
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG (2006) Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab 26:865–877
Occhipinti R, Somersalo E, Calvetti D (2009) Astrocytes as the glucose shunt for glutamatergic neurons at high activity: an in silico study. J Neurophysiol 101:2528–2538
Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL (2014) Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci USA 111:5385–5390
Chowdhury GM, Jiang L, Rothman DL, Behar KL (2014) The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J Cereb Blood Flow Metab 34(7):1233–1242s
Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M (2008) Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol 586:1337–1349
Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24:2784–2795
Hall CN, Klein-Flugge MC, Howarth C, Attwell D (2012) Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci 32:8940–8951
Howarth C, Gleeson P, Attwell D (2012) Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 32:1222–1232
McKenna MC, Tildon JT, Stevenson JH, Hopkins IB, Huang X, Couto R (1998) Lactate transport by cortical synaptosomes from adult rat brain: characterization of kinetics and inhibitor specificity. Dev Neurosci 20:300–309
O’Brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC (2007) Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res 32:597–607
Shank RP, Baldy WJ, Ash CW (1989) Glutamine and 2-oxoglutarate as metabolic precursors of the transmitter pools of glutamate and GABA: correlation of regional uptake by rat brain synaptosomes. Neurochem Res 14:371–376
McKenna MC, Stevenson JH, Huang X, Hopkins IB (2000) Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int 37:229–241
McKenna MC, Stevenson JH, Huang X, Tildon JT, Zielke CL, Hopkins IB (2000) Mitochondrial malic enzyme activity is much higher in mitochondria from cortical synaptic terminals compared with mitochondria from primary cultures of cortical neurons or cerebellar granule cells. Neurochem Int 36:451–459
McKenna MC, Hopkins IB, Lindauer SL, Bamford P (2006) Aspartate aminotransferase in synaptic and nonsynaptic mitochondria: differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation. Neurochem Int 48:629–636
Bogen IL, Risa O, Haug KH, Sonnewald U, Fonnum F, Walaas SI (2008) Distinct changes in neuronal and astrocytic amino acid neurotransmitter metabolism in mice with reduced numbers of synaptic vesicles. J Neurochem 105:2524–2534
Lai JCK, Clark JB (1989) Isolation and characterization of synaptic and nonsynaptic mitochondria from mammalian brain. Humana Press, Inc., Clifton, NJ
de Graaf RA, Brown PB, Mason GF, Rothman DL, Behar KL (2003) Detection of [1,6-13C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn Reson Med 49:37–46
Wang J, Jiang L, Jiang Y, Ma X, Chowdhury GM, Mason GF (2010) Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. J Neurochem 113:1447–1458
Waniewski RA, Martin DL (1998) Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci 18:5225–5233
Leke R, Bak LK, Schousboe A, Waagepetersen HS (2008) Demonstration of neuron-glia transfer of precursors for GABA biosynthesis in a co-culture system of dissociated mouse cerebral cortex. Neurochem Res 33:2629–2635
Patel AB, de Graaf RA, Rothman DL, Behar KL, Mason GF (2010) Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J Cereb Blood Flow Metab 30:1200–1213
Bradford HF, Ward HK, Thomas AJ (1978) Glutamine–a major substrate for nerve endings. J Neurochem 30:1453–1459
Reubi JC, Van Der Berg C, Cuenod M (1978) Glutamine as precursor for the GABA and glutamate trasmitter pools. Neurosci Lett 10:171–174
Battaglioli G, Martin DL (1996) Glutamine stimulates gamma-aminobutyric acid synthesis in synaptosomes but other putative astrocyte-to-neuron shuttle substrates do not. Neurosci Lett 209:129–133
Dienel GA, Cruz NF (2009) Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. J Neurochem 109(Suppl 1):30–37
Shen J, Rothman DL, Behar KL, Xu S (2009) Determination of the glutamate-glutamine cycling flux using two-compartment dynamic metabolic modeling is sensitive to astroglial dilution. J Cereb Blood Flow Metab 29:108–118
Schwartz WJ, Smith CB, Davidsen L, Savaki H, Sokoloff L, Mata M, Fink DJ, Gainer H (1979) Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 205:723–725
Sokoloff L (2005) Energy metabolism in neural tissues in vivo at rest and in functionally altered states. In: Brain Energetics and Neural Activity. John Wiley & Sons, Ltd., West Sussex, pp 11–30
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Kurumaji A, Nehls DG, Park CK, McCulloch J (1989) Effects of NMDA antagonists, MK-801 and CPP, upon local cerebral glucose use. Brain Res 496:268–284
Savaki HE, Desban M, Glowinski J, Besson MJ (1983) Local cerebral glucose consumption in the rat. I. Effects of halothane anesthesia. J Comp Neurol 213:36–45
Shapiro HM, Greenberg JH, Reivich M, Ashmead G, Sokoloff L (1978) Local cerebral glucose uptake in awake and halothane-anesthetized primates. Anesthesiology 48:97–103
Savaki HE, Desban M, Glowinski J, Besson MJ (1983) Local cerebral glucose consumption in the rat. II. Effects of unilateral substantia nigra stimulation in conscious and in halothane-anesthetized animals. J Comp Neurol 213:46–65
Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W (2008) Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci USA 105:6409–6414
Ben-Yoseph O, Camp DM, Robinson TE, Ross BD (1995) Dynamic measurements of cerebral pentose phosphate pathway activity in vivo using [1,6-13C2,6,6-2H2]glucose and microdialysis. J Neurochem 64:1336–1342
Gaitonde MK, Evison E, Evans GM (1983) The rate of utilization of glucose via hexosemonophosphate shunt in brain. J Neurochem 41:1253–1260
Hostetler KY, Landau BR (1967) Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry 6:2961–2964
Dienel GA, Cruz NF, Nakanishi H, Melzer P, Moulis P, Sokoloff L (1992) Comparison of rates of local cerebral glucose utilization determined with deoxy[1-14C]glucose and deoxy[6-14C]glucose. J Neurochem 59:1430–1436
Herzog RI, Jiang L, Herman P, Zhao C, Sanganahalli BG, Mason GF, Hyder F, Rothman DL, Sherwin RS, Behar KL (2013) Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest 123:1988–1998
Denker A, Bethani I, Krohnert K, Korber C, Horstmann H, Wilhelm BG, Barysch SV, Kuner T, Neher E, Rizzoli SO (2011) A small pool of vesicles maintains synaptic activity in vivo. Proc Natl Acad Sci USA 108:17177–17182
Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW (2001) Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci 24:637–643
Xue L, Sheng J, Wu XS, Wu W, Luo F, Shin W, Chiang HC, Wu LG (2013) Most vesicles in a central nerve terminal participate in recycling. J Neurosci 33:8820–8826
Waagepetersen HS, Qu H, Sonnewald U, Shimamoto K, Schousboe A (2005) Role of glutamine and neuronal glutamate uptake in glutamate homeostasis and synthesis during vesicular release in cultured glutamatergic neurons. Neurochem Int 47:92–102
Rimmele TS, Rosenberg PA (2016) GLT-1: the elusive presynaptic glutamate transporter. Neurochem Int 98:19–28
Schikorski T, Stevens CF (1997) Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci 17:5858–5867
Peng L, Hertz L, Huang R, Sonnewald U, Petersen SB, Westergaard N, Larsson O, Schousboe A (1993) Utilization of glutamine and of TCA cycle constituents as precursors for transmitter glutamate and GABA. Dev Neurosci 15:367–377
Laake JH, Takumi Y, Eidet J, Torgner IA, Roberg B, Kvamme E, Ottersen OP (1999) Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience 88:1137–1151
Bradford HF, Ward HK, Foley P (1989) Glutaminase inhibition and the release of neurotransmitter glutamate from synaptosomes. Brain Res 476:29–34
Conti F, Minelli A (1994) Glutamate immunoreactivity in rat cerebral cortex is reversibly abolished by 6-diazo-5-oxo-l-norleucine (DON), an inhibitor of phosphate-activated glutaminase. J Histochem Cytochem 42:717–726
Billups D, Marx MC, Mela I, Billups B (2013) Inducible presynaptic glutamine transport supports glutamatergic transmission at the calyx of Held synapse. J Neurosci 33:17429–17434
Patel AJ, Johnson AL, Balazs R (1974) Metabolic compartmentation of glutamate associated with the formation of gamma-aminobutyrate. J Neurochem 23:1271–1279
Battaglioli G, Martin DL (1990) Stimulation of synaptosomal gamma-aminobutyric acid synthesis by glutamate and glutamine. J Neurochem 54:1179–1187
Battaglioli G, Martin DL (1991) GABA synthesis in brain slices is dependent on glutamine produced in astrocytes. Neurochem Res 16:151–156
Patel AB, Rothman DL, Cline GW, Behar KL (2001) Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res 919:207–220
Sonnewald U, McKenna M (2002) Metabolic compartmentation in cortical synaptosomes: influence of glucose and preferential incorporation of endogenous glutamate into GABA. Neurochem Res 27:43–50
Larsson OM, Hertz L, Schousboe A (1986) Uptake of GABA and nipecotic acid in astrocytes and neurons in primary cultures: changes in the sodium coupling ratio during differentiation. J Neurosci Res 16:699–708
Scimemi A (2014) Structure, function, and plasticity of GABA transporters. Front Cell Neurosci 8:161
Manor D, Rothman DL, Mason GF, Hyder F, Petroff OA, Behar KL (1996) The rate of turnover of cortical GABA from [1-13C]-glucose is reduced in rats treated with the GABA-transaminase inhibitor vigabatrin (gamma-vinyl GABA). Neurochem Res 21:1031–1041
Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL (2001) Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD 67 protein. Brain Res 914:81–91
Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K (1997) Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 94:6496–6499
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166
Mangia S, Simpson IA, Vannucci SJ, Carruthers A (2009) The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem 109(Suppl 1):55–62
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81:89–131
Schwarz TL (2013) Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol 5. doi:10.1101/cshperspect.a011304
Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL (2014) Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell 158:54–68
Pfeuffer J, Tkac I, Gruetter R (2000) Extracellular-intracellular distribution of glucose and lactate in the rat brain assessed noninvasively by diffusion-weighted 1H nuclear magnetic resonance spectroscopy in vivo. J Cereb Blood Flow Metab 20:736–746
Choi IY, Gruetter R (2004) Dynamic or inert metabolism? Turnover of N-acetyl aspartate and glutathione from d-[1-13C]-glucose in the rat brain in vivo. J Neurochem 91:778–787
Acknowledgements
We thank Bei Wang for assistance with animal preparation, and Terry Nixon, Peter Brown and Scott McIntire for the maintenance of the NMR spectrometer and related equipment in the Yale Magnetic Resonance Research Center. The assistance of Dr. Graeme Mason in the implementation of the Monte-Carlo fitting routines within the CWave software was greatly appreciated.
Funding
Support for this work came, in part, from grants by the National Institutes of Health, NIDDK R01-DK027121 and NINDS R01-NS34813.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Dr. Behar discloses ownership of Pfizer common stock and consults for Merck. All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patel, A.B., Lai, J.C.K., Chowdhury, G.I.M. et al. Comparison of Glutamate Turnover in Nerve Terminals and Brain Tissue During [1,6-13C2]Glucose Metabolism in Anesthetized Rats. Neurochem Res 42, 173–190 (2017). https://doi.org/10.1007/s11064-016-2103-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2103-x