Abstract
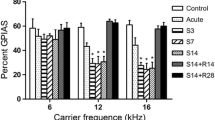
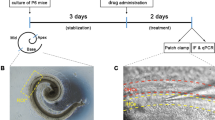
Lesion-induced cochlear damage can result in synaptic outgrowth in the ventral cochlear nucleus (VCN). Tinnitus may be associated with the synaptic outgrowth and hyperactivity in the VCN. However, it remains unclear how hearing loss triggers structural synaptic modifications in the VCN of rats subjected to salicylate-induced tinnitus. To address this issue, we evaluated tinnitus-like behavior in rats after salicylate treatment and compared the amplitude of the distortion product evoked otoacoustic emission (DPOAE) and auditory brainstem response (ABR) between control and treated rats. Moreover, we observed the changes in the synaptic ultrastructure and in the expression levels of growth-associated protein (GAP-43), brain-derived neurotrophic factor (BDNF), the microglial marker Iba-1 and glial fibrillary acidic protein (GFAP) in the VCN. After salicylate treatment (300 mg/kg/day for 4 and 8 days), analysis of the gap prepulse inhibition of the acoustic startle showed that the rats were experiencing tinnitus. The changes in the DPOAE and ABR amplitude indicated an improvement in cochlear sensitivity and a reduction in auditory input following salicylate treatment. The treated rats displayed more synaptic vesicles and longer postsynaptic density in the VCN than the control rats. We observed that the GAP-43 expression, predominantly from medial olivocochlear (MOC) neurons, was significantly up-regulated, and that BDNF- and Iba-1-immunoreactive cells were persistently decreased after salicylate administration. Furthermore, GFAP-immunoreactive astrocytes, which is associated with synaptic regrowth, was significantly increased in the treated groups. Our study revealed that reduced auditory nerve activity triggers synaptic outgrowth and hyperactivity in the VCN via a MOC neural feedback circuit. Structural synaptic modifications may be a reflexive process that compensates for the reduced auditory input after salicylate administration. However, massive increases in excitatory synapses in the VCN may represent a detrimental process that causes central hyperactivity, leading to tinnitus.






Similar content being viewed by others
References
Bauer CA (2004) Mechanisms of tinnitus generation. Curr Opin Otolaryngol Head Neck Surg 12:413–417
Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM (2000) GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147:251–260
Robertson D, Bester C, Vogler D, Mulders WH (2013) Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res 295:124–129
Vogler DP, Robertson D, Mulders WH (2011) Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci 31:6639–6645
Wallhausser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G (2003) Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res 153:649–654
Kraus KS, Ding D, Jiang H, Lobarinas E, Sun W, Salvi RJ (2011) Relationship between noise-induced hearing-loss, persistent tinnitus and growth-associated protein-43 expression in the rat cochlear nucleus: does synaptic plasticity in ventral cochlear nucleus suppress tinnitus. Neuroscience 194:309–325
Kraus KS, Ding D, Zhou Y, Salvi RJ (2009) Central auditory plasticity after carboplatin-induced unilateral inner ear damage in the chinchilla: up-regulation of GAP-43 in the ventral cochlear nucleus. Hear Res 255:33–43
Muly SM, Gross JS, Morest DK, Potashner SJ (2002) Synaptophysin in the cochlear nucleus following acoustic trauma. Exp Neurol 177:202–221
Illing RB, Horvath M, Laszig R (1997) Plasticity of the auditory brainstem: effects of cochlear ablation on GAP-43 immunoreactivity in the rat. J Comp Neurol 382:116–138
Muller M, Klinke R, Arnold W, Oestreicher E (2003) Auditory nerve fibre responses to salicylate revisited. Hear Res 183:37–43
Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. Trends Neurosci 27:676–682
Peng JH, Tao ZZ, Huang ZW (2007) Long-term sound conditioning increases distortion product otoacoustic emission amplitudes and decreases olivocochlear efferent reflex strength. NeuroReport 18:1167–1170
Yang K, Huang ZW, Liu ZQ, Xiao BK, Peng JH (2009) Long-term administration of salicylate enhances prestin expression in ratcochlea. Int J Audiol 48:18–23
Kraus KS, Illing RB (2004) Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J Comp Neurol 475:374–390
Benson TE, Berglund AM, Brown MC (1996) Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol 365:27–41
Fujino K, Oertel D (2001) Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21:7372–7383
Hanaya R, Boehm N, Nehlig A (2007) Dissociation of the immunoreactivity of synaptophysin and GAP-43 during the acute and latent phases of the lithium-pilocarpine model in the immature and adult rat. Exp Neurol 204:720–732
Kim SH, Kim MK, Yu HS, Kim HS, Park IS et al (2010) Electroconvulsive seizure increases phosphorylation of PKC substrates, including GAP-43, MARCKS, and neurogranin, in rat brain. Prog Neuropsychopharmacol Biol Psychiatry 34:115–121
Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K et al (2006) Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120:188–195
Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D et al (2007) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226:244–253
Brandt CT, Caye-Thomasen P, Lund SP, Worsoe L, Ostergaard C et al (2006) Hearing loss and cochlear damage in experimental pneumococcal meningitis, with special reference to the role of neutrophil granulocytes. Neurobiol Dis 23:300–311
Park CW, Lee JC, Ahn JH, Lee DH, Cho GS et al (2013) Neuronal damage using fluoro-Jade B histofluorescence and gliosis in the gerbils eptum submitted to various durations of cerebral ischemia. Cell Mol Neurobiol 33:991–1001
Friedland DR, Popper P, Eernisse R, Cioffi JA (2006) Differentially expressed genes in the rat cochlear nucleus. Neuroscience 142:753–768
McLaughlin S (1973) Salicylates and phospholipid bilayer membranes. Nature 243:234–236
Oliver D, He DZ, Klocker N, Ludwig J, Schulte U et al (2001) Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292:2340–2343
Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C et al (2010) Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265:63–69
Huang ZW, Luo Y, Wu Z, Tao Z, Jones RO, Zhao HB (2005) Paradoxical enhancement of active cochlear mechanics in long-term administration of salicylate. J Neurophysiol 93:2053–2061
Peng JH, Tao ZZ, Huang ZW (2007) Long-term sound conditioning increases distortion product otoacoustic emission amplitudes and decreases olivocochlear efferent reflex strength. NeuroReport 18:1167–1170
Favero ML, Sanchez TG, Bento RF, Nascimento AF (2006) Contralateral suppression of otoacoustic emission in patients with tinnitus. Braz J Otorhinolaryngol 72:223–226
Mulders WH, Seluakumaran K, Robertson D (2010) Efferent pathways modulate hyperactivity in inferior colliculus. J Neurosci 30:9578–9587
Illing RB, Kraus KS, Meidinger MA (2005) Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear Res 206:185–199
Kraus KS, Illing RB (2004) Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J Comp Neurol 475:374–390
Varghese GI, Zhu X, Frisina RD (2005) Age-related declines in distortion product otoacoustic emissions utilizing puretone contralateral stimulation in CBA/CaJ mice. Hear Res 209:60–67
Fujino K, Oertel D (2001) Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21:7372–7383
Cant NB, Benson CG (2003) Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull 60:457–474
Oertel D, Wright S, Cao XJ, Ferragamo M, Bal R (2011) The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res 276:61–69
Fujino K, Oertel D (2001) Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21:7372–7383
Kraus KS, Ding D, Jiang H, Kermany MH, Mitra S, Salvi RJ (2013) Up-regulation of GAP-43 in the chinchilla ventral cochlear nucleus after carboplatin-induced hearing loss: correlations with inner hair cell loss and outer hair cell loss. Hear Res 302:74–82
Mehrpouya S, Nahavandi A, Khojasteh F, Soleimani M, Ahmadi M, Barati M (2014) Iron administration prevents BDNF decrease and depressive-like behavior followingchronic stress. LID Brain Res S0006-8993(14)01479-6. doi:10.1016/j.brainres.2014.10.057
Mattson MP, Scheff SW (1994) Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma 11:3–33
Pardon MC (2010) Role of neurotrophic factors in behavioral processes: implications for the treatment of psychiatric and neurodegenerative disorders. Vitam Horm 82:185–200
Parpura V, Zorec R (2010) Gliotransmission: exocytotic release from astrocytes. Brain Res Rev 63:83–92
Trang T, Beggs S, Salter MW (2011) Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol 7:99–108
Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE et al (2006) Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31:149–160
Smith L, Gross J, Morest DK (2002) Fibroblast growth factors (FGFs) in the cochlear nucleus of the adult mouse following acoustic over stimulation. Hear Res 169:1–12
Feng J, Bendiske J, Morest DK (2012) Degeneration in the ventral cochlear nucleus after severe noise damage in mice. J Neurosci Res 90:831–841
Wang T, Wang SW, Zhang Y, Wu XF, Peng Y et al (2014) Scorpion venom heat-resistant peptide (SVHRP) enhances neurogenesis and neurite outgrowth of immature neurons in adult mice by up-regulating brain-derived neurotrophic factor (BDNF). PLoS ONE 9:e109977
Park KM, Bowers WJ (2010) Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 22:977–983
Hu SS, Mei L, Chen JY, Huang ZW, Wu H (2014) Effects of salicylate on the inflammatory genes expression and synaptic ultrastructure in the cochlear nucleus of rats. Inflammation 37:365–373
Hwang JH, Chen JC, Yang SY, Wang MF, Chan YC (2011) Expression of tumor necrosis factor-alpha and interleukin-1 beta genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation 8:30
Acknowledgments
This study was financed by the National Natural Science Fund Projects (Serial number 81070786), the Zhejiang Natural Science Fund Projects (Serial number LY16H130001), the Shanghai Health System Talents Training Program (XBR2011068) and the Shanghai Science and Technology Committee (12XD1401700).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, L., Fu, Y. & Zhang, Ty. Salicylate-Induced Hearing Loss Trigger Structural Synaptic Modifications in the Ventral Cochlear Nucleus of Rats via Medial Olivocochlear (MOC) Feedback Circuit. Neurochem Res 41, 1343–1353 (2016). https://doi.org/10.1007/s11064-016-1836-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1836-x