Abstract
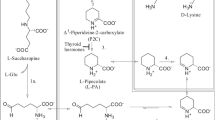
Mammalian ketimine reductase is identical to μ-crystallin (CRYM)—a protein that is also an important thyroid hormone binding protein. This dual functionality implies a role for thyroid hormones in ketimine reductase regulation and also a reciprocal role for enzyme catalysis in thyroid hormone bioavailability. In this research we demonstrate potent sub-nanomolar inhibition of enzyme catalysis at neutral pH by the thyroid hormones l-thyroxine and 3,5,3′-triiodothyronine, whereas other thyroid hormone analogues were shown to be far weaker inhibitors. We also investigated (a) enzyme inhibition by the substrate analogues pyrrole-2-carboxylate, 4,5-dibromopyrrole-2-carboxylate and picolinate, and (b) enzyme catalysis at neutral pH of the cyclic ketimines S-(2-aminoethyl)-l-cysteine ketimine (owing to the complex nomenclature trivial names are used for the sulfur-containing cyclic ketimines as per the original authors’ descriptions) (AECK), Δ1-piperideine-2-carboxylate (P2C), Δ1-pyrroline-2-carboxylate (Pyr2C) and Δ2-thiazoline-2-carboxylate. Kinetic data obtained at neutral pH suggests that ketimine reductase/CRYM plays a major role as a P2C/Pyr2C reductase and that AECK is not a major substrate at this pH. Thus, ketimine reductase is a key enzyme in the pipecolate pathway, which is the main lysine degradation pathway in the brain. In silico docking of various ligands into the active site of the X-ray structure of the enzyme suggests an unusual catalytic mechanism involving an arginine residue as a proton donor. Given the critical importance of thyroid hormones in brain function this research further expands on our knowledge of the connection between amino acid metabolism and regulation of thyroid hormone levels.






Similar content being viewed by others
Abbreviations
- AECK:
-
S-(2-Aminoethyl)-l-cysteine ketimine
- CE:
-
Catalytic efficiency
- CRYM:
-
μ-Crystallin
- CTBP:
-
Cytosolic thyroid hormone binding protein
- CysK:
-
Cystathionine ketmine
- DAAO:
-
d-Amino acid oxidase
- DIT:
-
3,5-Diiodo-l-tyrosine
- DTT:
-
Dithiothreitol
- LK:
-
Lanthionine ketimine
- L-PA:
-
l-Pipecolic acid
- P2C:
-
Δ1-Piperideine-2-carboxylate
- OTE:
-
Oxalyl thioester
- Pyr2C:
-
Δ1-Pyrroline-2-carboxylate
- SEM:
-
Standard error of the mean
- T2C:
-
Δ2-Thiazoline-2-carboxylate
- TL2C:
-
Thiazolidine-2-carboxylate
- T2 :
-
3,5-Diiodothyronine
- T3 :
-
3,5,3′-l-Triiodothyronine
- rT3 :
-
3,3′,5′-l-Triiodothyronine, reverse T3
- T4 :
-
l-Thyroxine
References
Hashizume K, Miyamoto T, Ichikawa K, Yamauchi K, Sakurai A, Ohtsuka H, Kobayashi M, Nishii Y, Yamada T (1989) Evidence for the presence of two active forms of cytosolic 3,5,3’-triiodo-L-thyronine (T3)-binding protein (CTBP) in rat kidney. Specialized functions of two CTBPs in intracellular T3 translocation. J Biol Chem 264(9):4864–4871
Segovia L, Horwitz J, Gasser R, Wistow G (1997) Two roles for μ-crystallin: a lens structural protein in diurnal marsupials and a possible enzyme in mammalian retinas. Mol Vis 3:9
Hallen A, Cooper AJ, Jamie JF, Haynes PA, Willows RD (2011) Mammalian forebrain ketimine reductase identified as μ-crystallin; potential regulation by thyroid hormones. J Neurochem 118(3):379–387
Nardini M, Ricci G, Caccuri AM, Solinas SP, Vesci L, Cavallini D (1988) Purification and characterization of a ketimine-reducing enzyme. Eur J Biochem 173(3):689–694
Nardini M, Ricci G, Vesci L, Pecci L, Cavallini D (1988) Bovine brain ketimine reductase. Biochim Biophys Acta 957(2):286–292
Lowry OH, Passonneau JV, Schulz DW, Rock MK (1961) The measurement of pyridine nucleotides by enzymatic cycling. J Biol Chem 236:2746–2755
Hallen A, Jamie JF, Cooper AJ (2014) Imine reductases: a comparison of glutamate dehydrogenase to ketimine reductases in the brain. Neurochem Res 39(3):527–541
Hallen A, Jamie JF, Cooper AJ (2013) Lysine metabolism in mammalian brain: an update on the importance of recent discoveries. Amino Acids 45(6):1249–1272
Petrakis PL, Greenberg DM (1965) Studies on l-proline:NAD(P)+-oxidoreductase of hog Kidney. Biochim Biophys Acta 99:78–95
Garweg G, von Rehren D, Hintze U (1980) l-Pipecolate formation in the mammalian brain. Regional distribution of Δ1-pyrroline-2-carboxylate reductase activity. J Neurochem 35(3):616–621
Visser WF, Verhoeven-Duif NM, de Koning TJ (2012) Identification of a human trans-3-hydroxy-L-proline dehydratase, the first characterized member of a novel family of proline racemase-like enzymes. J Biol Chem 287(26):21654–21662
Meister A, Radhakrishnan AN, Buckley SD (1957) Enzymatic synthesis of l-pipecolic acid and l-proline. J Biol Chem 229(2):789–800
Mestichelli LJG, Gupta RN, Spenser ID (1979) The biosynthetic route from ornithine to proline. J Biol Chem 254:640–647
Juncosa JI, Lee H, Silverman RB (2013) Two continuous coupled assays for ornithine-δ-aminotransferase. Anal Biochem 440(2):145–149
Radhakrishnan AN, Meister A (1957) Conversion of hydroxyproline to pyrrole-2-carboxylic acid. J Biol Chem 226(1):559–571
Naber N, Venkatesan PP, Hamilton GA (1982) Inhibition of dopamine β-hydroxylase by thiazoline-2-carboxylate, a suspected physiological product of d-amino acid oxidase. Biochem Biophys Res Commun 107(1):374–380
Fitzpatrick PF, Massey V (1982) Thiazolidine-2-carboxylic acid, an adduct of cysteamine and glyoxylate, as a substrate for d-amino acid oxidase. J Biol Chem 257(3):1166–1171
Afeefy HY, Hamilton GA (1987) Acetic anhydride in aqueous solution converts Δ2-thiazoline-2-carboxylate to an oxalyl thioester. Biol Chem 15:262–268
Cabello J, Leon B, Prajoux V, Plaza M (1964) Molecular changes during the titration of α-keto-δ-aminovaleric acid. Arch Biochem Biophys 107:51–56
Lu SP, Lewin AH (1998) Enamine/imine tautomerism in α, β-unsaturated-α-amino acids. Tetrahedron 54:15097–15104
Nishina Y, Sato K, Shiga K (1991) Isomerization of Δ1-piperideine-2-carboxylate to Δ2-piperideine-2-carboxylate on complexation with flavoprotein d-amino acid oxidase. J Biochem (Tokyo) 109(5):705–710
Lewis ML, Rowe CJ, Sewald N, Sutherland JD, Wilson EJ, Wright MC (1993) The effect of pH on the solution of structure of Δ1-pyrroline-2-carboxylate as revealed by NMR and mass spectrometry. Bioorg Med Chem Lett 3(6):1193–1196
Venkatasan PP, Hamilton GA (1986) Then nonenzymic hydrolysis of Δ2-thiazoline-2-carboxylate: the product of the suspected physiological reaction catalysed by d-amino acid oxidase. Bioorg Chem 14:392–404
Vie MP, Evrard C, Osty J, Breton-Gilet A, Blanchet P, Pomerance M, Rouget P, Francon J, Blondeau JP (1997) Purification, molecular cloning, and functional expression of the human nicodinamide-adenine dinucleotide phosphate-regulated thyroid hormone-binding protein. Mol Endocrinol 11(11):1728–1736
Beslin A, Vie MP, Blondeau JP, Francon J (1995) Identification by photoaffinity labelling of a pyridine nucleotide-dependent tri-iodothyronine-binding protein in the cytosol of cultured astroglial cells. Biochem J 305(Pt 3):729–737
Bernal J (2007) Thyroid hormone receptors in brain development and function. Nat Rev Endocrinol 3:249–259
Cavallini D, Ricci G, Federici G (1983) The ketimine derivatives of thialysine, lanthionine, cystathionine, cystine: preparation and properties. Prog Clin Biol Res 125:355–363
Szollosi G, Kun I, Bartok M (2001) Heterogeneous asymmetric reactions. Part 24. Heterogeneous catalytic enantioselective hydrogenation of the C=N group over cinchona alkaloid modified palladium catalyst. Chirality 13:619–624
Theodorou V, Skobridis K, Tzakos AG, Ragoussis V (2007) A simple method for the alkaline hydrolysis of esters. Tetrahedron Lett 48(46):8230–8233
Meister A (1954) The α-keto analogues of arginine, ornithine, and lysine. J Biol Chem 206(2):577–585
Liu W, Zhou J, Wong JT (1982) A novel synthesis of 3,5-diiodotyrosine with iodic acid. Anal Biochem 120(1):204–207
Richards JJ, Ballard TE, Huigens RW 3rd, Melander C (2008) Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem 9(8):1267–1279
Burch HB, Bradley ME, Lowry OH (1967) The measurement of triphosphopyridine nucleotide and reduced triphosphopyridine nucleotide and the role of hemoglobin in producing erroneous triphosphopyridine nucleotide values. J Biol Chem 242(19):4546–4554
Muramatsu H, Mihara H, Kakutani R, Yasuda M, Ueda M, Kurihara T, Esaki N (2005) The putative malate/lactate dehydrogenase from Pseudomonas putida is an NADPH-dependent Δ1-piperideine-2-carboxylate/Δ1-pyrroline-2-carboxylate reductase involved in the catabolism of d-lysine and d-proline. J Biol Chem 280(7):5329–5335
Morrison JF (1969) Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta 185(2):269–286
Borel F, Hachi I, Palencia A, Gaillard MC, Ferrer JL (2014) Crystal structure of mouse μ-crystallin complexed with NADPH and the T3 thyroid hormone. FEBS J 281(6):1598–1612
Srinivasan R, Fisher HF (1986) Structural features facilitating the glutamate dehydrogenase catalyzed α-imino acid-α-amino acid interconversion. Arch Biochem Biophys 246(2):743–750
Srinivasan R, Medary RT, Fisher HF, Norris DJ, Steward R (1982) The pyridinium-dihydropyridine system. Reduction potentials and the mechanism of oxidation of 1,4-dihydropyridines by a Schiff base. J Am Chem Soc 104:807–812
Cody V (1980) Role of iodine in thyroid hormones: molecular conformation of a halogen-free hormone analogue. J Med Chem 23(5):584–587
Okabe N, Fujiwara T, Yamagata Y, Tomita K (1982) The crystal structure of a major metabolite of thyroid hormone: 3,3′,5′-triiodo-l-thyronine. Biochim Biophys Acta 717(1):179–181
Campos-Barros A, Hoell T, Musa A, Sampaolo S, Stoltenburg G, Pinna G, Eravci M, Meinhold H, Baumgartner A (1996) Phenolic and tyrosyl ring iodothyronine deiodination and thyroid hormone concentrations in the human central nervous system. J Clin Endocrinol Metab 81(6):2179–2185
Pinna G, Meinhold H, Hiedra L, Thoma R, Hoell T, Graf KJ, Stoltenburg-Didinger G, Eravci M, Prengel H, Brodel O, Finke R, Baumgartner A (1997) Elevated 3,5-diiodothyronine concentrations in the sera of patients with nonthyroidal illnesses and brain tumors. J Clin Endocrinol Metab 82(5):1535–1542
Heacock AM, Adams E (1975) Formation and excretion of pyrrole-2-carboxylic acid. Whole animal and enzyme studies in the rat. J Biol Chem 250(7):2599–2608
Ebada SS, Edrada-Ebel R, de Voogd NJ, Wray V, Proksch P (2009) Dibromopyrrole alkaloids from the marine sponge Acanthostylotella sp. Nat Prod Commun 4(1):47–52
Leklem JE, Brown RR, Hankes LV, Schmaeler M (1971) Tryptophan metabolism in the cat: a study with carbon-14-labeled compounds. Am J Vet Res 32(2):335–344
Coggan SE, Smythe GA, Bilgin A, Grant RS (2009) Age and circadian influences on picolinic acid concentrations in human cerebrospinal fluid. J Neurochem 108(5):1220–1225
Goto M, Muramatsu H, Mihara H, Kurihara T, Esaki N, Omi R, Miyahara I, Hirotsu K (2005) Crystal structures of Δ1-piperideine-2-carboxylate/Δ1-pyrroline-2-carboxylate reductase belonging to a new family of NAD(P)H-dependent oxidoreductases: conformational change, substrate recognition, and stereochemistry of the reaction. J Biol Chem 280(49):40875–40884
Goodman JL, Wang S, Alam S, Ruzicka FJ, Frey PA, Wedekind JE (2004) Ornithine cyclodeaminase: structure, mechanism of action, and implications for the µ-crystallin family. Biochemistry 43(44):13883–13891
Meister A, Buckley SD (1957) Pyridine nucleotide-dependent reduction of the α-keto acid analogue of lysine to l-pipecolic acid. Biochim Biophys Acta 23(1):202–203
Chang YF (1976) Pipecolic acid pathway: the major lysine metabolic route in the rat brain. Biochem Biophys Res Commun 69(1):174–180
Warren GL, Andrews CW, Capelli AM, Clarke B, LaLonde J, Lambert MH, Lindvall M, Nevins N, Semus SF, Senger S, Tedesco G, Wall ID, Woolven JM, Peishoff CE, Head MS (2006) A critical assessment of docking programs and scoring functions. J Med Chem 49(20):5912–5931
Kim RY, Gasser R, Wistow GJ (1992) μ-Crystallin is a mammalian homologue of Agrobacterium ornithine cyclodeaminase and is expressed in human retina. Proc Natl Acad Sci USA 89(19):9292–9296
Schroder I, Vadas A, Johnson E, Lim S, Monbouquette HG (2004) A novel archaeal alanine dehydrogenase homologous to ornithine cyclodeaminase and μ-crystallin. J Bacteriol 186(22):7680–7689
Bergmeyer HU (1974) Methods of enzymatic analysis, Verlag Chemie Weinheim. Academic Press Inc, New York
Pinna G, Hiedra L, Prengel H, Broedel O, Eravci M, Meinhold H, Baumgartner A (1999) Extraction and quantification of thyroid hormones in selected regions and subcellular fractions of the rat brain. Brain Res Brain Res Protoc 4(1):19–28
Doherty MK, Pealing SL, Miles CS, Moysey R, Taylor P, Walkinshaw MD, Reid GA, Chapman SK (2000) Identification of the active site acid/base catalyst in a bacterial fumarate reductase: a kinetic and crystallographic study. Biochemistry 39(35):10695–10701
Francelle L, Galvan L, Gaillard MC, Guillermier M, Houitte D, Bonvento G, Petit F, Jan C, Dufour N, Hantraye P, Elalouf JM, De Chaldee M, Deglon N, Brouillet E (2015) Loss of the thyroid hormone-binding protein Crym renders striatal neurons more vulnerable to mutant huntingtin in Huntington’s disease. Hum Mol Genet 24(6):1563–1573
Fukada Y, Yasui K, Kitayama M, Doi K, Nakano T, Watanabe Y, Nakashima K (2007) Gene expression analysis of the murine model of amyotrophic lateral sclerosis: studies of the Leu126delTT mutation in SOD1. Brain Res 1160:1–10
Mistry M, Gillis J, Pavlidis P (2013) Genome-wide expression profiling of schizophrenia using a large combined cohort. Mol Psychiatry 18(2):215–225
Bohm C, Newrzella D, Herberger S, Schramm N, Eisenhardt G, Schenk V, Sonntag-Buck V, Sorgenfrei O (2006) Effects of antidepressant treatment on gene expression profile in mouse brain: cell type-specific transcription profiling using laser microdissection and microarray analysis. J Neurochem 97(Suppl 1):44–49
Acknowledgments
This research was funded by a Macquarie University Research Excellence Scholarship.
Conflict of interest
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hallen, A., Cooper, A.J.L., Jamie, J.F. et al. Insights into Enzyme Catalysis and Thyroid Hormone Regulation of Cerebral Ketimine Reductase/μ-Crystallin Under Physiological Conditions. Neurochem Res 40, 1252–1266 (2015). https://doi.org/10.1007/s11064-015-1590-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1590-5