Abstract
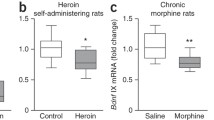
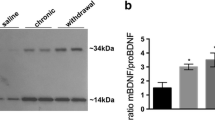
Brain-derived neurotrophic factor (BDNF) is believed to play a crucial role in the mechanisms underlying opiate dependence; however, little is known about specific features and mechanisms regulating its expression in the brain under these conditions. The aim of this study was to investigate the effects of acute morphine intoxication and withdrawal from chronic intoxication on expression of BDNF exon I-, II-, IV-, VI- and IX-containing transcripts in the rat frontal cortex and midbrain. We also have studied whether alterations of BDNF exon-specific transcripts are accompanied by changes in association of well-known transcriptional regulators of BDNF gene—phosphorylated (active form) cAMP response element binding protein (pCreb1) and methyl-CpG binding protein 2 (MeCP2) with corresponding regulatory regions of the BDNF gene. Acute morphine intoxication did not affect levels of BDNF exons in brain regions, while spontaneous morphine withdrawal in dependent rats was accompanied by an elevation of the BDNF exon I-containing mRNAs both in the frontal cortex and midbrain. During spontaneous morphine withdrawal, increased associations of pCreb1 were found with promoter of exon I in the frontal cortex and promoters of exon I, IV and VI in the midbrain. The association of MeCP2 with BDNF promoters during spontaneous morphine withdrawal did not change. Thus, BDNF exon-specific transcripts are differentially expressed in brain regions during spontaneous morphine withdrawal in dependent rats and pCreb1 may be at least partially responsible for these alterations.


Similar content being viewed by others
References
Kalivas PW, O’Brien C (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180
Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2:24–32
Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ (2009) Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 56:3–82
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258
Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ (1996) Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA 93:11202–11207
Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ (1995) Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience 68:969–979
Berhow MT, Hiroi N, Nestler EJ (1996) Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci 16:4707–4715
Vargas-Perez H, Ting-A Kee R, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D (2009) Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science 324:1732–1734
Numan S, Lane-Ladd SB, Zhang L, Lundgren KH, Russell DS, Seroogy KB, Nestler EJ (1998) Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci 18:10700–10708
Hatami H, Oryan S, Semnanian S, Kazemi B, Bandepour M, Ahmadiani A (2007) Alterations of BDNF and NT-3 genes expression in the nucleus paragigantocellularis during morphine dependency and withdrawal. Neuropeptides 41:321–328
Lunden JW, Kirby LG (2013) Opiate exposure and withdrawal dynamically regulate mRNA expression in the serotonergic dorsal raphe nucleus. Neuroscience 254:160–172
Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T (2007) Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85:525–535
Meng M, Zhao X, Dang Y, Ma J, Li L, Gu S (2013) Region-specific expression of brain-derived neurotrophic factor splice variants in morphine conditioned place preference in mice. Brain Res 1519:53–62
Matsushita Y, Ueda H (2009) Curcumin blocks chronic morphine analgesic tolerance and brain-derived neurotrophic factor upregulation. Neuroreport 20:63–68
Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, Chen J, Chi ZQ, He L, Liu JG (2012) Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci 32:13763–13775
Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH (2010) Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci 30:11735–11744
Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, Sadri-Vakili G (2012) Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem 120:202–209
Licata SC, Shinday NM, Huizenga MN, Darnell SB, Sangrey GR, Rudolph U, Rowlett JK, Sadri-Vakili G (2013) Alterations in brain-derived neurotrophic factor in the mouse hippocampus following acute but not repeated benzodiazepine treatment. PLoS One 8:e84806. doi:10.1371/journal.pone.008480
Lubin FD, Roth TL, Sweatt JD (2008) Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28:10576–10586
Duclot F, Kabbaj M (2013) Individual differences in novelty seeking predict subsequent vulnerability to social defeat through a differential epigenetic regulation of brain-derived neurotrophic factor expression. J Neurosci 33:11048–11060
Flavell SW, Greenberg ME (2008) Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31:563–590
Greer P, Greenberg M (2008) From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59:846–860
Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ (2002) Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci 22:3663–3672
Robinson TE, Kolb B (1999) Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33:160–162
Li Y, Wang H, Niu L, Zhou Y (2007) Chronic morphine exposure alters the dendritic morphology of pyramidal neurons in visual cortex of rats. Neurosci Lett 418:227–231
Spiga S, Serra GP, Puddu MC, Foddai M, Diana M (2003) Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur J Neurosci 17:605–612
Spiga S, Puddu MC, Pisano M, Diana M (2005) Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur J Neurosci 22:2332–2340
Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ (1997) Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 389:856–860
Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA (2007) Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci 26:2756–2763
Ali Khan R, Kumar A (2002) Experimental study of the morphine de-addiction properties of Delphinium denudatum Wall. BMC Complement Altern Med. doi:10.1186/1472-6882-2-6
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol chloroform extraction. Anal Biochem 162:156–159
Tsankova NM, Kumar A, Nestler EJ (2004) Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci 24:5603–5610
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE (2003) DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302:890–893
Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302:885–889
Jiang X, Tian F, Du Y, Copeland NG, Jenkins NA, Tessarollo L, Wu X, Pan H, Hu XZ, Xu K, Kenney H, Egan SE, Turley H, Harris AL, Marini AM, Lipsky RH (2008) BHLHB2 controls BDNF promoter 4 activity and neuronal excitability. J Neurosci 28:1118–1130
Riccio A (2010) Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci 13:1330–1337
Mashayekhi FJ, Rasti M, Rahvar M, Mokarram P, Namavar MR, Owji AA (2012) Expression levels of the BDNF gene and histone modifications around its promoters in the ventral tegmental area and locus ceruleus of rats during forced abstinence from morphine. Neurochem Res 37:1517–1523
Ciccarelli A, Calza A, Santoru F, Grasso F, Concas A, Sassoè-Pognetto M, Giustetto M (2013) Morphine withdrawal produces ERK-dependent and ERK-independent epigenetic marks in neurons of the nucleus accumbens and lateral septum. Neuropharmacology 70:168–179
Chiaruttini C, Sonego M, Baj G, Simonato M, Tongiorgi E (2008) BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Mol Cell Neurosci 37:11–19
Baj G, Leone E, Chao MV, Tongiorgi E (2011) Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proc Natl Acad Sci USA 108:16813–16818
Langerman L, Piscoun B, Bansinath M, Shemesh Y, Turndorf H, Grant GJ (2001) Quantifiable dose-dependent withdrawal after morphine discontinuation in a rat model. Pharmacol Biochem Behav 68:1–6
Rios M (2013) BDNF and the central control of feeding: accidental bystander or essential player? Trends Neurosci 36:83–90
Acknowledgment
The study was supported by Russian Foundation for Basic Research Grant No. 12-04-31478.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peregud, D.I., Panchenko, L.F. & Gulyaeva, N.V. Elevation of BDNF Exon I-Specific Transcripts in the Frontal Cortex and Midbrain of Rat During Spontaneous Morphine Withdrawal is Accompanied by Enhanced pCreb1 Occupancy at the Corresponding Promoter. Neurochem Res 40, 130–138 (2015). https://doi.org/10.1007/s11064-014-1476-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1476-y