Abstract
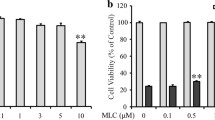
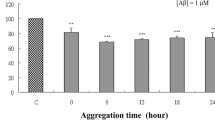
Neurofibrillary tangles are pathological hallmarks of Alzheimer’s disease (AD), which are mostly composed of hyperphosphorylated tau and directly correlate with dementia in AD patients. Okadaic acid (OA), a toxin extracted from marine life, can specifically inhibit protein phosphatases (PPs), including PP1 and Protein phosphatase 2A (PP2A), resulting in tau hyperphosphorylation. Humanin (HN), a peptide of 24 amino acids, was initially reported to protect neurons from AD-related cell toxicities. The present study was designed to test if HN could attenuate OA-induced neurotoxicities, including neural insults, apoptosis, autophagy, and tau hyperphosphorylation. We found that administration of OA for 24 h induced neuronal insults, including lactate dehydrogenase released, decreased of cell viability and numbers of living cells, neuronal apoptosis, cells autophagy and tau protein hyperphosphorylation. Pretreatment of cells with HN produced significant protective effects against OA-induced neural insults, apoptosis, autophagy and tau hyperphosphorylation. We also found that OA treatment inhibited PP2A activity and HN pretreatment significantly attenuated the inhibitory effects of OA. This study demonstrated for the first time that HN protected cortical neurons against OA-induced neurotoxicities, including neuronal insults, apoptosis, autophagy, and tau hyperphosphorylation. The mechanisms underlying the protections of HN may involve restoration of PP2A activity.







Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- NFTs:
-
Neurofibrillary tangles
- PHF:
-
Paired helical filaments
- Aβ:
-
Amyloid-β
- AVs:
-
Autophagic vacuoles
- PPs:
-
Protein phosphatases
- PP2A:
-
Protein phosphatase 2A
- OA:
-
Okadaic acid
- HN:
-
Humanin
- UP:
-
Unrelated peptide
- LDH:
-
Lactate dehydrogenase
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Ac-DEVD-pNA:
-
Acetyl-Asp-Glu-Val-Asp p-nitroaniline
- TUNEL:
-
Terminal-deoxynucleoitidyl transferase mediated nick end labeling
References
Vickers JC, Dickson TC, Adlard PA, Saunders HL, King CE, McCormack G (2000) The cause of neuronal degeneration in Alzheimer’s disease. Prog Neurobiol 60:139–165
Devred F, Barbier P, Douillard S, Monasterio O, Andreu JM, Peyrot V (2004) Tau induces ring and microtubule formation from alphabeta-tubulin dimers under nonassembly conditions. Biochemistry 43:10520–10531
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481
Del Barrio L, Martín-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J, McIntosh JM, Wonnacott S, López MG (2011) Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by α7 and β2* nicotinic stimulation. Toxicol Sci 123:193–205
Gong CX, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K (2000) Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer’s disease. J Biol Chem 275:5535–5544
Arendt T, Holzer M, Brückner MK, Janke C, Gärtner U (1998) The use of okadaic acid in vivo and the induction of molecular changes typical for Alzheimer’s disease. Neuroscience 85:1337–1340
Gong CX, Liu F, Grundke-Iqbal I, Iqbal K (2006) Dysregulation of protein phosphorylation/dephosphorylation in Alzheimer’s disease: a therapeutic target. J Biomed Biotechnol 2006:31825
Zhang Z, Simpkins JW (2010) An okadaic acid-induced model of tauopathy and cognitive deficiency. Brain Res 1359:233–246
Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2005) Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22:1942–1950
Liu GP, Zhang Y, Yao XQ, Zhang CE, Fang J, Wang Q, Wang JZ (2008) Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol Aging 29:1348–1358
Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Zhou XW, Wang Q, Wang DW, Wang JZ (2008) Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging 29:1654–1665
Cirak Y, Varol U, Atmaca H, Kisim A, Sezgin C, Karabulut B, Uzunoglu S, Uslu R, Karaca B (2012) Zoledronic acid in combination with serine/threonine phosphatase inhibitors induces enhanced cytotoxicity and apoptosis in hormone-refractory prostate cancer cell lines by decreasing the activities of PP1 and PP2A. BJU Int 110:E1147–E1154
Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL 3rd (2004) Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol 63:287–301
Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM (2001) PP2A mRNA expression is quantitatively decreased in Alzheimer’s disease hippocampus. Exp Neurol 168:402–412
Zhao ST, Huang XT, Zhang C, Ke Y (2012) Humanin protects cortical neurons from ischemia and reperfusion injury by the increased activity of superoxide dismutase. Neurochem Res 37:153–160
Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005) Extensive involvement of autophagy in Alzheimer disease: an immunoelectron microscopy study. J Neuropathol Exp Neurol 64:113–122
Yoon SY, Choi JE, Kweon HS, Choe H, Kim SW, Hwang O, Lee H, Lee JY, Kim DH (2008) Okadaic acid increases autophagosomes in rat neurons: implications for Alzheimer’s disease. J Neurosci Res 86:3230–3239
Ułamek-Kozioł M, Furmaga-Jabłońska W, Januszewski S, Brzozowska J, Sciślewska M, Jabłoński M, Pluta R (2013) Neuronal autophagy: self-eating or self-cannibalism in Alzheimer’s disease. Neurochem Res 38:1769–1773
Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11:709–730
Zhang J, Cai T, Zhao F, Yao T, Chen Y, Liu X, Luo W, Chen J (2012) The role of α-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci 8:935–944
Rosenfeldt MT, Ryan KM (2009) The role of autophagy in tumour development and cancer therapy. Expert Rev Mol Med 11:e36
Reggiori F, Klionsky DJ (2005) Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol 17:415–422
Sridhar S, Botbol Y, Macian F, Cuervo AM (2012) Autophagy and disease: always two sides to a problem. J Pathol 226:255–273
Sadasivan S, Zhang Z, Larner SF, Liu MC, Zheng W, Kobeissy FH, Hayes RL, Wang KK (2010) Acute NMDA toxicity in cultured rat cerebellar granule neurons is accompanied by autophagy induction and late onset autophagic cell death phenotype. BMC Neurosci 11:21
Niikura T, Hashimoto Y, Tajima H, Nishimoto I (2002) Death and survival of neuronal cells exposed to Alzheimer’s insults. J Neurosci Res 70:380–391
Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I (2001) A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA 98:6336–6341
Zapała B, Kaczyński Ł, Kieć-Wilk B, Staszel T, Knapp A, Thoresen GH, Wybrańska I, Dembińska-Kieć A (2010) Humanins, the neuroprotective and cytoprotective peptides with antiapoptotic and anti-inflammatory properties. Pharmacol Rep 62:767–777
Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, Jordan KL, Oh YK, Zhu XY, Korsmo MJ, Bachar AR, Cohen P, Lerman A, Lerman LO (2012) Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci 91:199–206
Kwon KJ, Kim HJ, Shin CY, Han SH (2010) Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating Amp-activated protein kinase pathways. J Clin Neurol 6:127–137
Amadoro G, Corsetti V, Ciotti MT, Florenzano F, Capsoni S, Amato G, Calissano P (2011) Endogenous Aβ causes cell death via early tau hyperphosphorylation. Neurobiol Aging 32:969–990
Guise S, Braguer D, Remacle-Bonnet M, Pommier G, Briand C (1999) Tau protein is involved in the apoptotic process induced by anti-microtubule agents on neuroblastoma cells. Apoptosis 4:47–58
Li X, Zhang X, Yuan H, Quan Q (2010) Experimental research on effect of gensenoside Rg1 on expressions of P-Tau and caspase-3 in brain slices from AD model rats. Zhongguo Zhong Yao Za Zhi 35:369–372
Furukawa K, D’Souza I, Crudder CH, Onodera H, Itoyama Y, Poorkaj P, Bird TD, Schellenberg GD (2000) Pro-apoptotic effects of tau mutations in chromosome 17 frontotemporal dementia and parkinsonism. NeuroReport 11:57–60
Hung KS, Hwang SL, Liang CL, Chen YJ, Lee TH, Liu JK, Howng SL, Wang CH (2005) Calpain inhibitor inhibits p35-p25-Cdk5 activation, decreases tau hyperphosphorylation, and improves neurological function after spinal cord hemisection in rats. J Neuropathol Exp Neurol 64:15–26
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA, Yu WH, Duff KE (2012) Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 8:609–622
Krüger U, Wang Y, Kumar S, Mandelkow EM (2011) Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol Aging 33:2291–2305
Pei JJ, Gong CX, An WL, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K (2003) Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer’s disease. Am J Pathol 163:845–858
Li LM, Zhang Y, Qiao JT, Zhang C (2010) Humanin protects neurons against apoptosis induced by Abeta31-35 through suppression of intrinsic pathway. Sheng Li Xue Bao 62:93–100
Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M (2009) Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell 20:2864–2873
Matsuoka M, Hashimoto Y (2010) Humanin and the receptors for humanin. Mol Neurobiol 41:22–28
Chiba T, Yamada M, Aiso S (2009) Targeting the JAK2/STAT3 axis in Alzheimer’s disease. Expert Opin Ther Targets 13:1155–1167
Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC (2003) Humanin peptide suppresses apoptosis by interfering with bax activation. Nature 423:456–461
Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC (2005) Humanin binds and nullifies bid activity by blocking its activation of bax and bak. J Biol Chem 280:15815–15824
Chiba T, Nishimoto I, Aiso S, Matsuoka M (2007) Neuroprotection against neurodegenerative diseases: development of a novel hybrid neuroprotective peptide colivelin. Mol Neurobiol 35:55–84
Kariya S, Hirano M, Furiya Y, Ueno S (2005) Effect of humanin on decreased ATP levels of human lymphocytes harboring A3243G mutant mitochondrial DNA. Neuropeptides 39:97–101
Liu Y, Zhang Y, Lin L, Lin F, Li T, Du H, Chen R, Zheng W, Liu N (2013) Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One 8:e78514
Veronesi MC, Yard M, Jackson J, Lahiri DK, Kubek MJ (2007) An analog of thyrotropin-releasing hormone (TRH) is neuroprotective against glutamate-induced toxicity in fetal rat hippocampal neurons in vitro. Brain Res 1128:79–85
Acknowledgments
The study was supported by National Natural Science Foundation of China (30572085).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jinfeng Zhao and Dan Wang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, J., Wang, D., Li, L. et al. Protective Effects of Humanin on Okadaic Acid-Induced Neurotoxicities in Cultured Cortical Neurons. Neurochem Res 39, 2150–2159 (2014). https://doi.org/10.1007/s11064-014-1410-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1410-3