Abstract
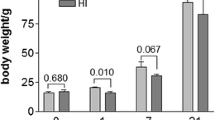
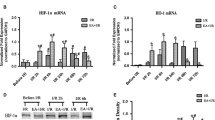
This study investigated the neuroprotection and potential mechanism of carbon monoxide (CO) against perinatal hypoxic–ischemic brain damage in rats by electrical acupuncture (EA). Animal behavior, morphological changes, cystathionine beta-synthase (CBS), hypoxia-inducible factor-1α (HIF-1α), and heme oxygenase-1 (HO-1) expression levels, and CO content in rat cortex cells were determined. Results demonstrated that EA treatment decreased the slope behavior and increased the overhang behavior of perinatal rats. The treatment also decreased the number of positive cells. The activator and inhibitor of CBS aggravated and remitted the hypoxic damage in cortex cells, respectively. EA treatment decreased CBS expression level and increased HO-1 and HIF-1α expression levels in perinatal rat cortex cells. Compared with the control groups, the CO content of cortex cells in the EA treatment group significantly increased (**p < 0.01). We hypothesized that EA treatment increases cortical CO content to protect against hypoxic damage via the hydrogen sulfide/CBS–CO/HO-1–HIF-1α system. This study provided a significant reference for EA therapy and cued a novel protective mechanism for cerebral palsy.






Similar content being viewed by others
References
Richards CL, Malouin F (2013) Cerebral palsy: definition, assessment and rehabilitation. Handb Clin Neurol 111:183–195
Hustad KC, Allison K, McFadd E, Riehle K (2014) Speech and language development in 2-year-old children with cerebral palsy. Dev Neurorehabil 17:167–175
Zivkovic Z, Golubovic S (2012) Tongue mobility in patients with cerebral palsy. Vojnosanit Pregl 69:488–491
Holmefur M, Kits A, Bergstrom J, Krumlinde-Sundholm L, Flodmark O, Forssberg H, Eliasson AC (2013) Neuroradiology can predict the development of hand function in children with unilateral cerebral palsy. Neurorehabil Neural Repair 27:72–78
Duncan B, Shen K, Zou LP, Han TL, Lu ZL, Zheng H, Walsh M, Venker C, Su Y, Schnyer R, Caspi O (2012) Evaluating intense rehabilitative therapies with and without acupuncture for children with cerebral palsy: a randomized controlled trial. Arch Phys Med Rehabil 93:808–815
Shi B, Bu H, Lin L (1992) A clinical study on acupuncture treatment of pediatric cerebral palsy. J Tradit Chin Med 12:45–51
Cavazos JE, Golarai G, Sutula TP (1991) Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci 11:2795–2803
Cavazos JE, Sutula TP (1990) Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res 527:1–6
Wu Y, Jin Z, Li K, Lu ZL, Wong V, Han TL, Zheng H, Caspi O, Liu G, Zeng YW, Zou LP (2008) Effect of acupuncture on the brain in children with spastic cerebral palsy using functional neuroimaging (FMRI). J Child Neurol 23:1267–1274
Liu Y, Zou LP, Du JB, Wong V (2010) Electro-acupuncture protects against hypoxic-ischemic brain-damaged immature rat via hydrogen sulfide as a possible mediator. Neurosci Lett 485:74–78
Liu Y, Zou LP, Du JB (2011) Nitric oxide-mediated neuronal functional recovery in hypoxic-ischemic brain damaged rats subjected to electrical stimulation. Brain Res 1383:324–328
Rice JE 3rd, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141
Semenza GL, Prabhakar NR (2012) Gas biology: small molecular medicine. J Mol Med (Berl) 90:213–215
Kajimura M, Nakanishi T, Takenouchi T, Morikawa T, Hishiki T, Yukutake Y, Suematsu M (2012) Gas biology: tiny molecules controlling metabolic systems. Respir Physiol Neurobiol 184:139–148
Hoshi T, Lahiri S (2004) Cell biology. Oxygen sensing: it’s a gas! Science 306:2050–2051
Suematsu M (2003) Quartet signal transducers in gas biology. Antioxid Redox Signal 5:435–437
Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS (2013) Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol 33:1104–1113
Zhang L, Yang G, Untereiner A, Ju Y, Wu L, Wang R (2013) Hydrogen sulfide impairs glucose utilization and increases gluconeogenesis in hepatocytes. Endocrinology 154:114–126
Zhang J, Chen S, Liu H, Zhang B, Zhao Y, Ma K, Zhao D, Wang Q, Ma H, Zhang Z (2013) hydrogen sulfide prevents hydrogen peroxide-induced activation of epithelial sodium channel through a PTEN/PI(3, 4, 5)P3 dependent pathway. PLoS One 8:e64304
Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R (2013) Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18:1906–1919
Tsuchiya M, Sato EF, Inoue M, Asada A (2007) Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth Analg 104:301–307
Ma SX, Li XY, Sakurai T, Pandjaitan M (2007) Evidence of enhanced non-enzymatic generation of nitric oxide on the skin surface of acupuncture points: an innovative approach in humans. Nitric Oxide 17:60–68
Zhao P, Huang ZN, Chen G, Cheng JS (2000) Electro-acupuncture attenuates nitric oxide release from rat striatum after transient middle cerebral artery occlusion. Acupunct Electrother Res 25:101–107
Acknowledgments
This research projects is sponsored by Supported by: International Science and Technology Cooperation Fundation of the Ministry of Science and Technology of China, (No.2008DFA31850), International Cooperation of Science and Technique Foundation of Beijing (2007G05) and the Beijing Chinese medicine projects (Grant no. JJ2005-17).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yichen Liu is the first author.
Rights and permissions
About this article
Cite this article
Liu, Y., Li, Z., Shi, X. et al. Neuroprotection of Up-Regulated Carbon Monoxide by Electrical Acupuncture on Perinatal Hypoxic–Ischemic Brain Damage in Rats. Neurochem Res 39, 1724–1732 (2014). https://doi.org/10.1007/s11064-014-1366-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1366-3