Abstract
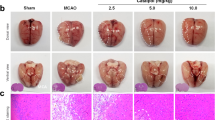
Inflammatory damage plays a pivotal, mainly detrimental role in cerebral ischemic pathogenesis and may represent a promising target for treatment. Naringenin (NG) has gained growing appreciation for its beneficial biological effects through its anti-inflammatory property. Whether this protective effect applies to cerebral ischemic injury, we therefore investigate the potential neuroprotective role of NG and the underlying mechanisms. Focal cerebral ischemia in male Sprague–Dawley rats was induced by permanent middle cerebral artery occlusion (pMCAO) and NG was pre-administered intragastrically once daily for four consecutive days before surgery. Neurological deficit, brain water content and infarct volume were measured at 24 h after stroke. Immunohistochemistry, Western blot and RT-qPCR were used to explore the anti-inflammatory potential of NG in the regulation of NOD2, RIP2 and NF-κB in ischemic cerebral cortex. Additionally, the activities of MMP-9 and claudin-5 were analyzed to detect NG’s influence on blood–brain barrier. Compared with pMCAO and Vehicle groups, NG noticeably improved neurological deficit, decreased infarct volume and edema at 24 h after ischemic insult. Consistent with these results, our data also indicated that NG significantly downregulated the expression of NOD2, RIP2, NF-κB and MMP-9, and upregulated the expression of claudin-5 (P < 0.05). The results provided a neuroprotective profile of NG in cerebral ischemia, this effect was likely exerted by down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression.






Similar content being viewed by others
References
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J (2010) American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 121:948–954
Perera MN, Ma HK, Arakawa S, Howells DW, Markus R, Rowe CC, Donnan GA (2006) Inflammation following stroke. J Clin Neurosci 13:1–8
Liu Y, Zhang X, Yang C, Fan HG (2009) Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-κB expression. Brain Res 1268:174–180
Fan H, Li L, Zhang X, Liu Y, Yang C, Yang Y, Yin J (2009) Oxymatrine downregulates TLR4, TLR2, MyD88, and NF-κB and protects rat brains against focal ischemia. Mediat Inflamm 2009:704706
Cui L, Zhang X, Yang R, Wang L, Liu L, Li M, Du W (2010) Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Res 1325:164–173
Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Núñez G (2007) RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol 178:2380–2386
Shigeoka AA, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J, Ulevitch RJ, McKay DB (2010) Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J Immunol 184:2297–2304
Li H, Hu J, Ma L, Yuan Z, Wang Y, Wang X, Xing D, Lei F, Du L (2010) Comprehensive study of baicalin down-regulating NOD2 receptor expression of neurons with oxygen–glucose deprivation in vitro and cerebral ischemia-reperfusion in vivo. Eur J Pharmacol 649:92–99
Abbott DW, Wilkins A, Asara JM, Cantley LC (2004) The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol 14:2217–2227
Mattson MP, Camandola S (2001) NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107:247–254
Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R (2001) Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci 68:921–931
Renugadevi J, Prabu SM (2009) Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 256:128–134
Mojzisová G, Sarisský M, Mirossay L, Martinka P, Mojzis J (2009) Effect of flavonoids on daunorubicin-induced toxicity in H9c2 cardiomyoblasts. Phytother Res 23:136–139
Shi Y, Dai J, Liu H, Li RR, Sun PL, Du Q, Pang LL, Chen Z, Yin KS (2009) Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can J Physiol Pharmacol 87:729–735
Tsai SJ, Huang CS, Mong MC, Kam WY, Huang HY, Yin MC (2012) Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J Agric Food Chem 60:514–521
Jayaraman J, Jesudoss VA, Menon VP, Namasivayam N (2012) Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods 22:568–576
Tsai TH (2002) Determination of naringin in rat blood, brain, liver, and bile using microdialysis and its interaction with cyclosporine A, a P-glycoprotein modulator. J Agric Food Chem 50:6669–6674
Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (2003) Interaction between flavonoids and the blood–brain barrier: in vitro studies. J Neurochem 85:180–192
Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39:1119–1125
Heo HJ, Kim DO, Shin SC, Kim MJ, Kim BG, Shin DH (2004) Effect of antioxidant flavanone, naringenin, from Citrus. junoson neuroprotection. J Agric Food Chem 52:1520–1525
Raza SS, Khan MM, Ahmad A, Ashafaq M, Islam F, Wagner AP, Safhi MM, Islam F (2013) Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience 230:157–171
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Yang C, Zhang X, Fan H, Liu Y (2009) Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282:133–141
Ding Y, Li J, Rafols JA, Phillis JW, Diaz FG (2002) Prereperfusion saline infusion into ischemic territory reduces inflammatory injury after transient middle cerebral artery occlusion in rats. Stroke 33:2492–2498
Tatlisumak T, Carano RA, Takano K, Opgenorth TJ, Sotak CH, Fisher M (1998) A novel endothelin antagonist, A-127722, attenuates ischemic lesion size in rats with temporary middle cerebral artery occlusion: a diffusion and perfusion MRI study. Stroke 29:850–857
Ikeda K, Negishi H, Yamori Y (2003) Antioxidant nutrients and hypoxia/ischemia brain injury in rodents. Toxicology 189:55–61
Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY (2005) Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol 31:135–147
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14
Wang L, Zhang X, Liu L, Cui L, Yang R, Li M, Du W (2010) Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-κB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res 1321:143–151
Qiao H, Zhang X, Zhu C, Dong L, Wang L, Zhang X, Xing Y, Wang C, Ji Y, Cao X (2012) Luteolin downregulates TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res 1448:71–81
Inohara N, Nuñez G (2001) The NOD: a signaling module that regulates apoptosis and host defense against pathogens. Oncogene 20:6473–6481
Carneiro LA, Magalhaes JG, Tattoli I, Philpott DJ, Travassos LH (2008) Nod-like proteins in inflammation and disease. J Pathol 214:136–148
Tolle LB, Standiford TJ (2013) Danger associated molecular patterns (DAMPs) in acute lung injury. J Pathol 229:145–156
Body-Malapel M, Dharancy S, Berrebi D, Louvet A, Hugot JP, Philpott DJ, Giovannini M, Chareyre F, Pages G, Gantier E, Girardin SE, Garcia I, Hudault S, Conti F, Sansonetti PJ, Chamaillard M, Desreumaux P, Dubuquoy L, Mathurin P (2008) NOD2: a potential target for regulating liver injury. Lab Invest 88:318–327
McCarthy JV, Ni J, Dixit VM (1998) RIP2 is a novel NF-kappaB-activating and cell death-inducing kinase. J Biol Chem 273:16968–16975
Kobayashi K, Inohara N, Hernandez LD, Galán JE, Núñez G, Janeway CA, Medzhitov R, Flavell RA (2002) RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194–199
Sinha B, Yano H, Li W, Wang X, Friedlander RM (2011) Receptor inducing protein-2 (RIP-2) deficiency is neuroprotective against hypoxic-ischemic brain injury in newborn mice. Pediatr Res 70:66
Inohara N, del Peso L, Koseki T, Chen S, Nunez G (1998) RICK, a novel protein kinase containing a caspase recruitment domain, interacts with CLARP and regulates CD95-mediated apoptosis. J Biol Chem 273:12296–12300
Thome M, Hofmann K, Burns K, Martinon F, Bodmer JL, Mattmann C, Tschopp J (1998) Identification of CARDIAK, a RIP-like kinase that associates with caspase-1. Curr Biol 8:885–888
Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G (2002) Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416:190–194
Małek R, Borowicz KK, Jargiełło M, Czuczwar SJ (2007) Role of nuclear factor kappaB in the central nervous system. Pharmacol Rep 59:25–33
Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J (2004) Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke 35:987–991
Zhang HL, Gu ZL, Savitz SI, Han F, Fukunaga K, Qin ZH (2008) Neuroprotective effects of prostaglandinA(1) in rat models of permanent focal cerebral ischemia are associated with nuclear factor-kappaB inhibition and peroxisome proliferator-activated receptor-gamma up-regulation. J Neurosci Res 86:1132–1141
Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709
Mark KS, Davis TP (2002) Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 282:H1485–H1494
Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194
Zhang L, Zhao H, Zhang X, Chen L, Zhao X, Bai X, Zhang J (2013) Nobiletin protects against cerebral ischemia via activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and ameliorating BBB permeability in rat. Brain Res Bull 96:45–53
Wang L, Li Z, Zhang X, Wang S, Zhu C, Miao J, Chen L, Cui L, Qiao H (2013) Protective effect of shikonin in experimental ischemic stroke: attenuated TLR4, p-p38MAPK, NF-κB, TNF-α and MMP-9 expression, up-regulated claudin-5 expression, ameliorated BBB permeability. Neurochem Res 39:97–106
Acknowledgments
This work was funded by the National Natural Science Foundation of China (Grant No. 81371287) and Hebei Province (Grant Nos. C2010000564 and 10276104D). We thank technicians Ruichun Liu and Hongran Wu for their technical assistance and Prof. Yansu Guo M.D. PhD. and Weisong Duan M.D. PhD. for providing valuable suggestions.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, X., Zhang, X., Chen, L. et al. Protective Effect of Naringenin in Experimental Ischemic Stroke: Down-Regulated NOD2, RIP2, NF-κB, MMP-9 and Up-Regulated Claudin-5 Expression. Neurochem Res 39, 1405–1415 (2014). https://doi.org/10.1007/s11064-014-1326-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1326-y