Abstract
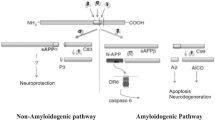
Chronic administration of aluminium has been proposed as an environmental factor that may affect some pathological changes related to neurotoxicity and Alzheimer’s disease (AD). The abnormal generation and deposition of β-amyloid (Aβ) in senile plaques are hallmark features in the brains of AD patients. Furthermore, Aβ is generated by the sequential cleavage of the amyloid precursor protein (APP) via the APP cleaving enzyme (α-secretase, or β-secretase) and γ-secretase. In the present study, we investigated the modulation of Aβ deposition and neurotoxicity in aluminium-maltolate-treated (0, 15, 30, 45 mmol/kg body weight via intraperitoneal injection) in experimental rats. We measured Aβ1–40 and Aβ1–42 in the cortex and hippocampus in rat brains using ELISA. Subtypes of α-secretase, β-secretase, and γ-secretase, including ADAM9, ADAM10, ADAM17 (TACE), BACE1, presenilin 1 (PS1) and nicastrin (NCT), were determined using western blotting analyses. These results indicated that aluminium-maltolate induced an AD-like behavioural deficit in rats at 30 and 45 mmol/kg body weight. Moreover, the Aβ1–42 content increased significantly, both in the cortex and hippocampus, although no changes were observed in Aβ1–40. Furthermore, ADAM9, ADAM10, and ADAM17 decreased significantly; in contrast, BACE1, PS1, and NCT showed significant increase. Taken together, these results suggest that the changes in secretases may correlate to the abnormal deposition of Aβ by aluminium in rat brains.




Similar content being viewed by others
References
Wu G, Sankaranarayanan S, Hsieh SH, Simon AJ, Savage MJ (2011) Decrease in brain soluble amyloid precursor protein beta (sAPPbeta) in Alzheimer’s disease cortex. J Neurosci Res 89:822–832
Jiang T, Zhi XL, Zhang YH, Pan LF, Zhou P (2012) Inhibitory effect of curcumin on the Al(III)-induced Abeta(4)(2) aggregation and neurotoxicity in vitro. Biochim Biophys Acta 1822:1207–1215
Castorina A, Tiralongo A, Giunta S, Carnazza ML, Scapagnini G, D’Agata V (2010) Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of beta-amyloid toxicity. Cell Biol Toxicol 26:367–377
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8:79–84
Banks WA, Niehoff ML, Drago D, Zatta P (2006) Aluminum complexing enhances amyloid beta protein penetration of blood-brain barrier. Brain Res 1116:215–221
Walton JR (2013) Aluminum involvement in the progression of Alzheimer’s disease. J Alzheimers Dis (JAD) 35:7–43
Brenner S (2013) Aluminum may mediate Alzheimer’s disease through liver toxicity, with aberrant hepatic synthesis of ceruloplasmin and ATPase7B, the resultant excess free copper causing brain oxidation, beta-amyloid aggregation and Alzheimer disease. Med Hypotheses 80:326–327
Walton JR (2012) Cognitive deterioration and associated pathology induced by chronic low-level aluminum ingestion in a translational rat model provides an explanation of Alzheimer’s disease, tests for susceptibility and avenues for treatment. Int J Alzheimers Dis 2012:914947
Liang RF, Li WQ, Wang XH, Zhang HF, Wang H, Wang JX, Zhang Y, Wan MT, Pan BL, Niu Q (2012) Aluminium-maltolate-induced impairment of learning, memory and hippocampal long-term potentiation in rats. Ind Health 50:428–436
Ferreira PC, Piai Kde A, Takayanagui AM, Segura-Munoz SI (2008) Aluminum as a risk factor for Alzheimer’s disease. Rev Lat Am Enfermagem 16:151–157
Ricchelli F, Drago D, Filippi B, Tognon G, Zatta P (2005) Aluminum-triggered structural modifications and aggregation of beta-amyloids. Cell Mol Life Sci (CMLS) 62:1724–1733
Drago D, Folin M, Baiguera S, Tognon G, Ricchelli F, Zatta P (2007) Comparative effects of Abeta(1-42)-Al complex from rat and human amyloid on rat endothelial cell cultures. J Alzheimers Dis (JAD) 11:33–44
Zaky A, Mohammad B, Moftah M, Kandeel KM, Bassiouny AR (2013) Apurinic/apyrimidinic endonuclease 1 is a key modulator of aluminum-induced neuroinflammation. BMC Neurosci 14:26
Fiorelli T, Kirouac L, Padmanabhan J (2013) Altered processing of amyloid precursor protein in cells undergoing apoptosis. PLoS One 8:e57979
Deuss M, Reiss K, Hartmann D (2008) Part-time alpha-secretases: the functional biology of ADAM 9, 10 and 17. Curr Alzheimer Res 5:187–201
Allinson TM, Parkin ET, Turner AJ, Hooper NM (2003) ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74:342–352
Lichtenthaler SF (2011) Alpha-secretase in Alzheimer’s disease: molecular identity, regulation and therapeutic potential. J Neurochem 116:10–21
Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D, Ward PJ (1990) Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science (New York, NY) 248:1122–1124
Roberts SB, Ripellino JA, Ingalls KM, Robakis NK, Felsenstein KM (1994) Non-amyloidogenic cleavage of the beta-amyloid precursor protein by an integral membrane metalloendopeptidase. J Biol Chem 269:3111–3116
Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F (1999) Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA 96:3922–3927
Delarasse C, Auger R, Gonnord P, Fontaine B, Kanellopoulos JM (2011) The purinergic receptor P2X7 triggers alpha-secretase-dependent processing of the amyloid precursor protein. J Biol Chem 286:2596–2606
Frykman S, Hur JY, Franberg J, Aoki M, Winblad B, Nahalkova J, Behbahani H, Tjernberg LO (2010) Synaptic and endosomal localization of active gamma-secretase in rat brain. PLoS One 5:e8948
Smolarkiewicz M, Skrzypczak T, Wojtaszek P (2013) The very many faces of presenilins and the γ-secretase complex. Protoplasma 250:997–1011
Dries DR, Yu G (2008) Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr Alzheimer Res 5:132–146
Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM (2005) Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging 26:645–654
Langui D, Probst A, Anderton B, Brion JP, Ulrich J (1990) Aluminium-induced tangles in cultured rat neurones. Enhanced effect of aluminium by addition of maltol. Acta Neuropathol 80:649–655
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55:187–196
Martyn CN (1992) The epidemiology of Alzheimer’s disease in relation to aluminium. Ciba Found Symp Ciba 169:69–79 discussion 79–86
Chen TJ, Hung HS, Wang DC, Chen SS (2010) The protective effect of Rho-associated kinase inhibitor on aluminum-induced neurotoxicity in rat cortical neurons. Toxicol Sci 116:264–272
Bhalla P, Dhawan DK (2009) Protective role of lithium in ameliorating the aluminium-induced oxidative stress and histological changes in rat brain. Cell Mol Neurobiol 29:513–521
Kaur A, Joshi K, Minz RW, Gill KD (2006) Neurofilament phosphorylation and disruption: a possible mechanism of chronic aluminium toxicity in Wistar rats. Toxicology 219:1–10
Campbell A, Kumar A, La Rosa FG, Prasad KN, Bondy SC (2000) Aluminum increases levels of beta-amyloid and ubiquitin in neuroblastoma but not in glioma cells. Proc Soc Exp Biol Med (New York, NY) 223:397–402
Yumoto S, Kakimi S, Ohsaki A, Ishikawa A (2009) Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer’s disease. J Inorg Biochem 103:1579–1584
Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM (2002) Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J 16:1138–1140
Sun ZZ, Chen ZB, Jiang H, Li LL, Li EG, Xu Y (2009) Alteration of Abeta metabolism-related molecules in predementia induced by AlCl3 and D-galactose. Age 31:277–284
Marcinkiewicz M, Seidah NG (2000) Coordinated expression of beta-amyloid precursor protein and the putative beta-secretase BACE and alpha-secretase ADAM10 in mouse and human brain. J Neurochem 75:2133–2143
Skovronsky DM, Fath S, Lee VM, Milla ME (2001) Neuronal localization of the TNFalpha converting enzyme (TACE) in brain tissue and its correlation to amyloid plaques. J Neurobiol 49:40–46
Harada H, Tamaoka A, Ishii K, Shoji S, Kametaka S, Kametani F, Saito Y, Murayama S (2006) Beta-site APP cleaving enzyme 1 (BACE1) is increased in remaining neurons in Alzheimer’s disease brains. Neurosci Res 54:24–29
Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, Logan S, Maus E, Citron M, Berry R, Binder L, Vassar R (2007) Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J Neurosci 27:3639–3649
Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M (2005) Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem 92:628–636
Li Q, Sudhof TC (2004) Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J Biol Chem 279:10542–10550
Gustaw KA, Garrett MR, Lee HG, Castellani RJ, Zagorski MG, Prakasam A, Siedlak SL, Zhu X, Perry G, Petersen RB, Friedland RP, Smith MA (2008) Antigen-antibody dissociation in Alzheimer disease: a novel approach to diagnosis. J Neurochem 106:1350–1356
Panahi N, Mahmoudian M, Mortazavi P, Hashjin GS (2013) Effects of berberine on beta-secretase activity in a rabbit model of Alzheimer’s disease. Arch Med Sci (AMS) 9:146–150
Hass MR, Sato C, Kopan R, Zhao G (2009) Presenilin: RIP and beyond. Semin Cell Dev Biol 20:201–210
Chen F, Yu G, Arawaka S, Nishimura M, Kawarai T, Yu H, Tandon A, Supala A, Song YQ, Rogaeva E, Milman P, Sato C, Yu C, Janus C, Lee J, Song L, Zhang L, Fraser PE, St George-Hyslop PH (2001) Nicastrin binds to membrane-tethered Notch. Nat Cell Biol 3:751–754
Dries DR, Shah S, Han YH, Yu C, Yu S, Shearman MS, Yu G (2009) Glu-333 of nicastrin directly participates in gamma-secretase activity. J Biol Chem 284:29714–29724
Pasternak SH, Bagshaw RD, Guiral M, Zhang S, Ackerley CA, Pak BJ, Callahan JW, Mahuran DJ (2003) Presenilin-1, nicastrin, amyloid precursor protein, and gamma-secretase activity are co-localized in the lysosomal membrane. J Biol Chem 278:26687–26694
LaVoie MJ, Fraering PC, Ostaszewski BL, Ye W, Kimberly WT, Wolfe MS, Selkoe DJ (2003) Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J Biol Chem 278:37213–37222
Zhang YW, Luo WJ, Wang H, Lin P, Vetrivel KS, Liao F, Li F, Wong PC, Farquhar MG, Thinakaran G, Xu H (2005) Nicastrin is critical for stability and trafficking but not association of other presenilin/gamma-secretase components. J Biol Chem 280:17020–17026
Mao G, Cui MZ, Li T, Jin Y, Xu X (2012) Pen-2 is dispensable for endoproteolysis of presenilin 1, and nicastrin-Aph subcomplex is important for both gamma-secretase assembly and substrate recruitment. J Neurochem 123:837–844
Sesele K, Thanopoulou K, Paouri E, Tsefou E, Klinakis A, Georgopoulos S (2013) Conditional inactivation of nicastrin restricts amyloid deposition in an Alzheimer’s disease mouse model. Aging cell 12:1032–1040
Acknowledgments
This work was supported by the National Nature Science Foundation of China (81302410).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Hu, J., Zhao, Y. et al. Effects of Aluminium on β-Amyloid (1–42) and Secretases (APP-Cleaving Enzymes) in Rat Brain. Neurochem Res 39, 1338–1345 (2014). https://doi.org/10.1007/s11064-014-1317-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1317-z