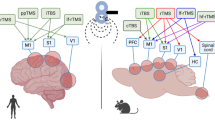
In a group of healthy adult male albino rats (n=6), we measured pain thresholds under conditions of electrocutaneous stimulation of the limbs (in a chamber with an electrified floor). The animals were subjected to the action of a modeled baroacoustic wave (excess pressure 26–36 kPa) leading to mild blast-induced traumatic brain injury (bTBI). It was found that such a trauma resulted in a long-lasting (up to four weeks) decrease in the above threshold (i.e., in an increase in the sensitivity of the nociceptive system) estimated according to the minimum intensity (μA) of 50-Hz alternating stimulation current evoking a pain-related behavioral response (vocalization). The pain threshold was measured at repeated stimulations of the increasing intensity of animals under light inhalation (halothane) anesthesia. There were reasons to believe that such an effect included two phases, an early (up to three days) and a later more long-lasting phase. The dynamics of the pain threshold in the bTBI group of rats were compared with those in the groups of fully intact rats (intact group) and rats subjected to the procedures of inhalation anesthesia and soft fixation but with no action of the baroacoustic wave (sham group). It is concluded that even mild blast-related trauma leads to significant long-lasting changes in the functioning of the nociceptive and antinociceptive brain neuronal systems, especially in their opioid-mediated components. These shifts develop due to energy deficiency, oxidative stress, and the accompanying mitochondrial damage. Such findings confirm suppositions that blast trauma-related changes in the cerebral opioid systems play a considerable role in the disorders of brain cognitive functions disturbed because of a blast-induced brain injury.
Similar content being viewed by others
References
M. Govindarajulu, M. Y. Patel, D. M. Wilder, et al., “Upregulation of multiple toll-like receptors in ferret brain after blast exposure: Potential targets for treatment,” Neurosci. Lett., 27, 810 (2023); doi: https://doi.org/10.1016/j.neulet.2023.137364.
A. Aravind, A. R. Ravula, N. Chandra, and B. J. Pfister, “Behavioral deficits in animal models of blast traumatic brain injury,” Front. Neurol., 11, 990 (2020); doi: https://doi.org/10.3389/fneur.2020.00990.
M. R. Dickerson, Z. S. Bailey, S. F. Murphy, et al., “Glial activation in the thalamus contributes to vestibulomotor deficits following blast-induced neurotrauma,” Front. Neurol., 11, 618 (2020); doi: https://doi.org/10.3389/fneur.2020.00618. PMID: 32760340; PMCID: PMC7373723.
P. S. Nabity, C. A. Jaramillo, P. A. Resick, et al., “Persistent posttraumatic headaches and functioning in veterans: Injury type can matter,” Headache, 61, No. 9, 1334–1341 (2021); doi: https://doi.org/10.1111/head.14210.
S. Bouferguène, A. Lapierre, B. Houzé, et al., “Chronic central pain among community-dwelling survivors of moderate-to-severe traumatic brain injury: a quantitative sensory testing study,” Biol. Res. Nurs., 21, No. 5, 519–531 (2019); doi: https://doi.org/10.1177/1099800419859078.
L. M. Anderson, S. Samineni, D. M. Wilder, et al., “The neurobehavioral effects of Buprenorphine and Meloxicam on a blast-induced traumatic brain injury model in the rat,” Front. Neurol., 12, 746370 (2021); doi: https://doi.org/10.3389/fneur.2021.746370.
S. A. Armstrong and M. J. Herr, “Physiology, Nociception,” In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023).
K. N. Gurba, R. Chaudhry, and S. Haroutounian, “Central neuropathic pain syndromes: current and emerging pharmacological strategies,” CNS Drugs, 36, No. 5, 483–516 (2022); doi: https://doi.org/10.1007/s40263-022-00914-4.
M. A. Fitzcharles, S. P. Cohen, D. J. Clauw, et al., “Nociplastic pain: towards an understanding of prevalent pain conditions,” Lancet, 397, No. 10289, P2098–2110 (2021); doi: https://doi.org/10.1016/S0140-6736(21)00392-5.
B. Stefanowski, A. Antosik-Wójcińska, and Ł. Święcicki, “The use of buprenorphine in the treatment of drug-resistant depression - an overview of the studies,” Psychiatr. Pol., 54, No. 2, 199–207 (2020); doi: https://doi.org/10.12740/PP/102658.
E. Duque-Díaz, O. Alvarez-Ojeda, and R. Coveñas, “Enkephalins and ACTH in the mammalian nervous system,” Vitam. Horm., 111, 147–193 (2019); doi: https://doi.org/10.1016/bs.vh.2019.05.001.
E. G. Miranda, V. P. Nascimento, D. R. Waisberg, et al., “Inhalation anesthesia equipment for rats with provision of simultaneous anesthetic and oxygen,” Acta Cir. Bras., 26, No. 2, 140–143 (2011); doi: https://doi.org/10.1590/s0102-86502011000200012.
Yu. V. Kozlova, Device for Studying the Effect of the Shock Wave of an Explosion on the Body, Utility model patent No. 146858 U, bul. No. 12, 24.03.2021.
M. Vincler, W. Maixner, C. J. Vierck, and A. R. Light, “Estrous cycle modulation of nociceptive behaviors elicited by electrical stimulation and formalin,” Pharmacol. Biochem. Behav., 69, No. 3–4, 315–324 (2001); doi: https://doi.org/10.1016/s0091-3057(01)00506-8.
J. P. Lefaucheur, “Clinical neurophysiology of pain,” Handb. Clin. Neurol., 161, 121–148 (2019); doi: https://doi.org/10.1016/B978-0-444-64142-7.00045-X.
D. De Ridder, D. Adhia, and S. Vanneste, “The anatomy of pain and suffering in the brain and its clinical implications,” Neurosci. Biobehav. Rev., 130, 125–146 (2021); doi: https://doi.org/10.1016/j.neubiorev.2021.08.013.
Yu. V. Kozlova and S. V. Kozlov, “Changes of trace elements in cerebellum and their influence on the rats behavior in elevated plus maze in the acute period of mild blast-induced brain injury,” J. Trace Elem. Med. Biol., 78, 127189 (2023); doi:https://doi.org/10.1016/j.jtemb.2023.127189.
H. C, Shih, J. W. Yang, C. M. Lee, and B. C. Shyu, “Spontaneous cingulate high-current spikes signal normal and pathological pain states,” J. Neurosci., 39, No. 26, 5128–5142 (2019); doi: https://doi.org/10.1523/JNEUROSCI.2590-18.2019.
E. Freye, “Endogenous opioids: endorphins and enkephalins” In: Opioid Agonists, Antagonists and Mixed Narcotic Analgesics. Springer, Berlin, Heidelberg (1987); doi:https://doi.org/10.1007/978-3-642-71854-0_8.
M. Zhou, Q. Zhang, M. Huo, et al., “The mechanistic basis for the effects of electroacupuncture on neuropathic pain within the central nervous system,” Biomed. Pharmacother., 161, 114516 (2023); doi: https://doi.org/10.1016/j.biopha.2023.114516.
I. Chen and F. Lui, “Neuroanatomy, neuron action potential,” In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546639/ (2023).
G. Corder, D. C. Castro, M. R. Bruchas, and G. Scherrer, “Endogenous and exogenous opioids in pain,” Annu. Rev. Neurosci., 41, 453–473 (2018); doi: https://doi.org/10.1146/annurevneuro-080317-061522.
B Dwyer, and N. Zasler, “Post-traumatic cephalalgia,” NeuroRehabilitation, 47, No. 3, 327–342 (2020); doi: https://doi.org/10.3233/NRE-208006.
M. Nazıroğlu, A. Öz, and K. Yıldızhan, “Selenium and neurological diseases: focus on peripheral pain and TRP channels,” Curr. Neuropharmacol., 18, No. 6, 501–517 (2020); doi: https://doi.org/10.2174/1570159X18666200106152631.
K. Nakamoto and S. Tokuyama, “Stress-induced changes in the endogenous opioid system cause dysfunction of pain and emotion regulation,” Int. J. Mol. Sci., 24, No. 14, 11713 (2023); doi: https://doi.org/10.3390/ijms241411713.
M. Kuriakose, D. Younger, A. R. Ravula, et al., “Synergistic role of oxidative stress and blood-brain barrier permeability as injury mechanisms in the acute pathophysiology of blast-induced neurotrauma,” Sci. Rep., 9, No. 1, 7717 (2019); doi: https://doi.org/10.1038/s41598-019-44147-w.
Q. X. Shi, B. Chen, C. Nie, et al., “Improvement in cognitive dysfunction following blast induced traumatic brain injury by thymosin α1 in rats: Involvement of inhibition of tau phosphorylation at the Thr205 epitope,” Brain Res., 1747, 147038 (2020); doi: https://doi.org/10.1016/j.brainres.2020.147038.
B. Varastehmoradi, G. Wegener, C. Sanchez, and K. L. Smith, “Opioid system modulation of cognitive affective bias: implications for the treatment of mood disorders,” Behav. Pharmacol., 31, No. 2–3, 122–135 (2020); doi: https://doi.org/10.1097/FBP.0000000000000559.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kozlova, Y.V., Demchenko, O.M. Excitability of the Nociceptive System in Rats after Blast-Induced Traumatic Brain Injury. Neurophysiology (2024). https://doi.org/10.1007/s11062-024-09945-7
Received:
Published:
DOI: https://doi.org/10.1007/s11062-024-09945-7