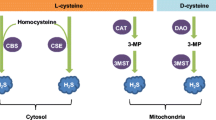
The literature data and results of our research team concerning the physiological and pathological effects of hydrogen sulfide, a gas transmitter that has recently attracted significant and increasing attention of neurophysiologists, are analyzed. Hydrogen sulfide is a gaseous signaling molecule, the effects of which were discovered later than those of NO and CO; H2S was found to play great pathophysiological roles in various diseases. This compound is synthesized in the body by enzymatic and non-enzymatic pathways from cysteine, using the pyridoxal 5’-phosphate-dependent enzymes cystathionine β-synthase (CBS) or cystathionine γ-lyase (CSE); 3-mercaptopyruvate sulfurtransferase (3MST) plays a considerable role in H2S catabolism. Abnormal deviations in H2S metabolism are important factors involved in the development of a number of dangerous neurological pathologies, in particular of Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), and Down’s syndrome (DS). This compound was also demonstrated to be involved in various biological and pathophysiological processes in the brain resulting from traumatic brain injury (TBI), stroke, oxidative stress, and cerebral edema. In the case of traumatic injury in the trout brain, the activation of CBS expression in the radial glia phenotypes occurs in the aNSC.
Similar content being viewed by others
References
X. Che, Y. Fang, X. Si, et al., “The role of gaseous molecules in traumatic brain injury: an updated review,” Front. Neurosci., 12, 392 (2018). doi: https://doi.org/10.3389/fnins.2018.00392.
J. Zhou, P. F. Wu, F. Wang, and J. G. Chen, “Targeting gaseous molecules to protect against cerebral ischaemic injury: mechanisms and prospects,” Clin. Exp. Pharmacol. Physiol., 39, No. 6, 566–576 (2012). doi: https://doi.org/10.1111/j.1440-1681.2011.05654.x.
J. Deng, C. Lei, Y. Chen, et al., “Neuroprotective gases – fantasy or reality for clinical use?,” Prog. Neurobiol., 115, 210–245 (2014). doi: https://doi.org/10.1016/j.pneurobio.2014.01.001.
U. Förstermann and W. C. Sessa, “Nitric oxide synthases: regulation and function,” Eur. Heart J., 33, No. 7, 829–837 (2012). doi: https://doi.org/10.1093/eurheartj/ehr304.
E. Galea, D. L. Feinstein, and D. J. Reis, “Induction of calcium-independent nitric oxide synthase activity in primary rat glial cultures,” Proc. Natl. Acad. Sci. U.S.A., 89, No. 22, 10945–10949 (1992). doi: https://doi.org/10.1073/pnas.89.22.10945
R. Olivenza, M. A. Moro, I. Lizasoain, et al., “Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex,” J. Neurochem., 74, No. 2, 785–791 (2000). doi: https://doi.org/10.1046/j.1471-4159.2000.740785.x.
W. Cai, H. Liu, J. Zhao, et al., “Pericytes in brain injury and repair after ischemic stroke,” Transl. Stroke Res., 8, No. 2, 107–121 (2017). doi: https://doi.org/10.1007/s12975-016-0504-4.
H. Parfenova, and C. W. Leffler, “Cerebroprotective functions of HO-2,” Curr. Pharm. Des., 14, No. 5, 443–453 (2008). doi: https://doi.org/10.2174/138161208783597380
H. Liu, Y. Wang, Y. Xiao, et al., “Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke,” Transl. Stroke Res., 7, No. 3, 209–219 (2016). doi: https://doi.org/10.1007/s12975-016-0459-5.
M. Ishigami, K. Hiraki, K. Umemura, et al., “A source of hydrogen sulfide and a mechanism of its release in the brain,” Antioxid. Redox Signal., 11, No. 2, 205–214 (2009). doi: https://doi.org/10.1089/ars.2008.2132.
N. Shibuya, M. Tanaka, M. Yoshida, et al., “3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain,” Antioxid. Redox Signal., 11, No. 4, 703–714 (2009). doi: https://doi.org/10.1089/ars.2008.2253.
R. Wang, “Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter?,” FASEB J., 16, No. 13, 1792–1798 (2002). doi: https://doi.org/10.1096/fj.02-0211hyp.
E. Łowicka and J. Bełtowski, “Hydrogen sulfide (H2S) – the third gas of interest for pharmacologists,” Pharmacol. Rep., 59, No. 1, 4–24 (2007).
R. Wang, “Shared signaling pathways among gasotransmitters,” Proc. Natl. Acad. Sci. U.S.A., 109, No. 23, 8801–8802 (2012). doi: https://doi.org/10.1073/pnas.1206646109.
Y. Liu, R. Yang, X. Liu, et al., “Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration,” Cell Stem Cell, 15, No. 1, 66–78. (2014). doi: https://doi.org/10.1016/j.stem.2014.03.005.
R. F. Furchgott and D. Jothianandan, “Endotheliumdependent and independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light,” Blood Vessels, 28, Nos. 1–3, 52–61 (1991).
P. Mergenthaler, U. Dirnagl, and A. Meisel, “Pathophysiology of stroke: lessons from animal models,” Metab. Brain Dis., 19, Nos. 3–4, 151–167 (2004). doi: https://doi.org/10.1023/b:mebr.0000043966.46964.e6.
G. Cirino, V. Vellecco, and M. Bucci, “Nitric oxide and hydrogen sulfide: the gasotransmitter paradigm of the vascular system,” Br. J. Pharmacol., 174, No. 22, 4021–4031 (2017). doi: https://doi.org/10.1111/bph.13815.
S. Panthi, S. Manandhar, and K. Gautam, “Hydrogen sulfide, nitric oxide, and neurodegenerative disorders,” Transl. Neurodegener., 7, 3 (2018). doi: https://doi.org/10.1186/s40035-018-0108-x.
S. Panthi, H.-J. Chung, J. Jung, and N. Y. Jeong, “Physiological importance of hydrogen sulfide: emerging potent neuroprotector and neuromodulator”, Oxid. Med. Cell. Longev., 2016, 9049782 (2016). doi: https://doi.org/10.1155/2016/9049782.
C. Coletta, A. Papapetropoulos, K. Erdelyi, et al., “Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation,” Proc. Natl. Acad. Sci. U.S.A., 109, No. 23, 9161–9166 (2012). doi: https://doi.org/10.1073/pnas.1202916109.
S. Taoka and R. Banerjee, “Characterization of NO binding to human cystathionine β-synthase: Possible implications of the effects of CO and NO binding to the human enzyme,” J. Inorg. Biochem., 87, No. 4, 245–251 (2001). doi: https://doi.org/10.1016/s0162-0134(01)00335-x.
J. Bełtowskil and A. Jamroz-Wiśniewska, “Hydrogen sulfide and endothelium-dependent vasorelaxation,” Molecules, 19, No. 12, 21506–21528 (2014). doi: https://doi.org/10.3390/molecules191221183.
H. Kimura, Y. Nagai, K. Umemura, and Y. Kimura, “Physiological roles of hydrogen sulfide: synaptic modulation, neuroprotection, and smooth muscle relaxation,” Antioxid. Redox Signal., 7, Nos. 5–6, 795–803 (2005). doi: https://doi.org/10.1089/ars.2005.7.795.
J. Zhang, Y. Ding, Z. Wang, et al., “Hydrogen sulfide therapy in brain diseases: from bench to bedside,” Med. Gas. Res., 7, No. 2, 113–119 (2017). doi: https://doi.org/10.4103/2045-9912.208517.
K. Qu, S. W. Lee, J. S. Bian, et al., “Hydrogen sulfide: neurochemistry and neurobiology,” Neurochem. Int., 52, Nos. 1–2, 155–165 (2008). doi: https://doi.org/10.1016/j.neuint.2007.05.016.
X. Chen, K. H. Jhee, and W. D. Kruger, “Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine,” J. Biol. Chem., 279, No. 50, 52082–52086 (2004). doi: https://doi.org/10.1074/jbc.C400481200.
Y. Mikami, N. Shibuya, Y. Kimura, et al., “Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide,” Biochem. J., 439, No. 3, 479–485 (2011). doi: https://doi.org/10.1042/BJ20110841.
S. Tang, D. Huang, N. An, et al., “A novel pathway for the production of H2S by DAO in rat jejunum,” Neurogastroenterol Motil., 28, No. 5, 687–692 (2016). doi: https://doi.org/10.1111/nmo.12765.
D. J. Polhemus and D. J. Lefer, “Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease,” Circ. Res., 114, No. 4, 730–737 (2014). doi: https://doi.org/10.1161/CIRCRESAHA.114.300505.
A. Hermann, G. F. Sitdikova, and T. M. Weiger, Gasotransmitters: Physiology and Pathophysiology, Springer, 163–201 (2012).
B. H. Tan, P. T.-H. Wong, and J.-S. Bian, “Hydrogen sulfide: a novel signaling molecule in the central nervous system,” Neurochem. Int., 56, No. 1, 3–10 (2010). doi: https://doi.org/10.1016/j.neuint.2009.08.008.
K. Kida and F. Ichinose, “Hydrogen sulfide and neuroinflammation,” Handb. Exp. Pharmacol., 230, 181–189 (2015). doi: https://doi.org/10.1007/978-3-319-18144-8_9.
L. Xie, L.-F. Hu, X. Q. Teo, et al., “Therapeutic effect of hydrogen sulfide-releasing L-Dopa derivative ACS84 on 6-OHDA-induced Parkinson’s disease rat model,” PLoS One, 8, No. 4, e60200. (2013). doi: https://doi.org/10.1371/journal.pone.0060200.
X. Cao, L. Cao, L. Ding, and J. Bian, “A new hope for a devastating disease: hydrogen sulfide in Parkinson’s disease,” Mol. Neurobiol., 55, No. 5, 3789–3799 (2017).
A. Xuan, D. Long, J. Li, et al., “Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in β-amyloid rat model of Alzheimer’s disease,” J. Neuroinflammation, 9, 202 (2012). doi: https://doi.org/10.1186/1742-2094-9-202.
K. Eto, T. Asada, K. Arima, et al., “Brain hydrogen sulfide is severely decreased in Alzheimer’s disease,” Biochem. Biophys. Res. Commun., 293, No. 5, 1485–1488 (2002). doi: https://doi.org/10.1016/S0006-291X(02)00422-9.
L.-M. Zhang, C.-X. Jiang, and D.-W. Liu, “Hydrogen sulfide attenuates neuronal injury induced by vascular dementia via inhibiting apoptosis in rats,” Neurochem. Res., 34, No. 11, 1984–1992 (2009). doi: https://doi.org/10.1007/s11064-009-0006-9.
D. Giuliani, A. Ottani, D. Zaffe, et al., “Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms,” Neurobiol. Learn. Mem., 104, 82–91 (2013). doi: https://doi.org/10.1016/j.nlm.2013.05.006.
B. D. Paul, J. I. Sbodio, R. Xu, et al., “Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease,” Nature, 509, No. 7498, 96–100 (2014). doi: https://doi.org/10.1038/nature13136.
H. Kimura, N. Shibuya, and Y. Kimura, “Hydrogen sulfide is a signaling molecule and a cytoprotectant,” Antioxid. Redox Signal., 17, No. 1, 45–57 (2012). doi: https://doi.org/10.1089/ars.2011.4345.
S. W. Lee, Y.-S. Hu, L.-F. Hu, et al., “Hydrogen sulphide regulates calcium homeostasis in microglial cells,” Glia, 54, No. 2, 116–124 (2006). doi: https://doi.org/10.1002/glia.20362.
J. F. Wang, Y. Li, J. N. Song, and H. G. Pang, “Role of hydrogen sulfide in secondary neuronal injury,” Neurochem. Int., 64, 37–47 (2014). doi: https://doi.org/10.1016/j.neuint.2013.11.002.
A. G. Mustafa and O. A. Alshboul, “Pathophysiology of traumatic brain injury,” Neurosciences (Riyadh), 18, No. 3, 222–234 (2013).
S. A. Karimi, N. Hosseinmardi, M. Janahmadi, et al., “The protective effect of hydrogen sulfide (H2S) on traumatic brain injury (TBI) induced memory deficits in rats,” Brain Res. Bull., 134, 177–182 (2017). doi: https://doi.org/10.1016/j.brainresbull.2017.07.014.
M. Zhang, H. Shan, T. Wang, et al., “Dynamic change of hydrogen sulfide after traumatic brain injury and its effect in mice,” Neurochem. Res., 38, No. 4, 714–725 (2013). doi: https://doi.org/10.1007/s11064-013-0969-4.
Q.-J. Chu, L. He, W. Zhang, et al., “Hydrogen sulfide attenuates surgical trauma-induced inflammatory response and cognitive deficits in mice,” J. Surg. Res., 183, No. 1, 330–336 (2013).
S. W. Scheff, M. A. Ansari, and K. N. Roberts, “Neuroprotective effect of Pycnogenol(R) following traumatic brain injury,” Exp. Neurol., 239, 183–191 (2013). doi: https://doi.org/10.1016/j.expneurol.2012.09.019.
R. Wang, “Physiological implication of hydrogen sulfide: a whiff exploration that blossomed,” Physiol. Rev., 92, No. 2, 791–896 (2012).
X. Jiang, Y. Huang, W. Lin, et al., “Protective effects of hydrogen sulfide in a rat model of traumatic brain injury via activation of mitochondrial adenosine triphosphate-sensitive potassium channels and reduction of oxidative stress,” J. Surg. Res. 184, No. 2, e27–e35 (2013). doi: https://doi.org/10.1016/j.jss.2013.03.067.
M. Zhang, H. Shan, P. Chang, et al., “Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice,” PLoS One, 9, No. 1, e87241 (2014). doi: https://doi.org/10.1371/journal.pone.0087241
K. Qu, C. P. Chen, B. Halliwell, et al., “Hydrogen sulfide is a mediator of cerebral ischemic damage,” Stroke, 37, No. 3, 889–893 (2006). doi: https://doi.org/10.1161/01.STR.0000204184.34946.41.
C. W. Leffler, H. Parfenova, S. Basuroy, et al., “Hydrogen sulfide and cerebral microvascular tone in newborn pigs,” Am. J. Physiol. Heart. Circ. Physiol., 300, No. 2, H440–H447 (2011). doi: https://doi.org/10.1152/ajpheart.00722.2010.
G. H. Liang, A. Adebiyi, M. D. Leo, et al., “Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels,” Am. J. Physiol. Heart Circ. Physiol., 300, No. 6, H2088–H2095 (2011). doi: https://doi.org/10.1152/ajpheart.01290.2010.
T. Kalogeris, C. P Baines, M. Krenz, and R. J. Korthuis, “Cell biology of ischemia/reperfusion injury,” Int. Rev. Cell Mol. Biol., 298, 229–317 (2012). doi: https://doi.org/10.1016/B978-0-12-394309-5.00006-7.
K. Abe and H. Kimura, “The possible role of hydrogen sulfide as an endogenous neuromodulator,” J. Neurosci., 16, No. 3, 1066–1071 (1996). doi: https://doi.org/10.1523/JNEUROSCI.16-03-01066.1996.
Y. Nagai, M. Tsugane, J.-I. Oka, and H. Kimura, “Hydrogen sulfide induces calcium waves in astrocytes,” FASEB J., 18, No. 3, 557–559 (2004). doi: https://doi.org/10.1096/fj.03-1052fje.
M. Lee, C. Schwab, S. Yu, E. McGeer, and P. L. Mc-Geer, “Astrocytes produce the anti-inflammatory and neuroprotective agent hydrogen sulfide,” Neurobiol. Aging, 30, No. 10, 1523–1534 (2009). doi: https://doi.org/10.1016/j.neurobiolaging.2009.06.001.
P. Nagy and C. Winterbourn, “Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides,” Chem. Res. Toxicol., 23, No. 10, 1541–1543 (2010). doi: https://doi.org/10.1021/tx100266a.
Y. Kimura, Y. Mikami, K. Osumi, et al., “Polysulfides are possible H2S-derived signaling molecules in rat brain,” FASEB J., 27, No. 6, 2451–2457 (2013). doi: https://doi.org/10.1096/fj.12-226415.
H. Kimura, ‘Physiological role of hydrogen sulfide and polysulfide in the central nervous system,” Neurochem. Int., 63, No. 5, 492–497 (2013). doi: https://doi.org/10.1016/j.neuint.2013.09.003.
P. Gopalakrishnan, B. Shrestha, A. M. Kaskas, et al., “Hydrogen sulfide: therapeutic or injurious in ischemic stroke?,” Pathophysiology, 26, No. 1, 1–10 (2019). doi: https://doi.org/10.1016/j.pathophys.2018.10.005.
Y. Kimura, R. Dargusch, D. Schubert, and H. Kimura, “Hydrogen sulfide protects HT22 neuronal cells from oxidative stress,” Antioxid. Redox Signal., 8, Nos. 3–4, 661–670 (2006). doi: https://doi.org/10.1089/ars.2006.8.661.
L. Xiao, A. Lan, L. Mo, et al., “Hydrogen sulfide protects PC12 cells against reactive oxygen species and extracellular signal-regulated kinase 1/2-mediated downregulation of glutamate transporter-1 expression induced by chemical hypoxia,” Int. J. Mol. Med., 30, No. 5, 1126–1132 (2012). doi: https://doi.org/10.3892/ijmm.2012.1090.
N. S. Cheung, Z. F. Peng, M. J. Chen, et al., “Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons,” Neuropharmacology, 53, No. 4, 505–514 (2007). doi: https://doi.org/10.1016/j.neuropharm.2007.06.014.
J. Wu, J. D. Holstein, G. Upadhyay, et al., “Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging,” J. Neurosci., 27, No. 24, 6510–6520 (2007). doi: https://doi.org/10.1523/JNEUROSCI.1256-07.2007.
M. Fu, W. Zhang, L. Wu, et al., “Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production,” Proc. Natl. Acad. Sci. U.S.A., 109, No. 8, 2943–2948 (2012). doi: https://doi.org/10.1073/pnas.1115634109.
Y. Luo, X. Yang, S. Zhao, et al., “Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROSmediated caspase-3 pathway in cortical neurons,” Neurochem. Int., 63, No. 8, 826–831 (2013). doi: https://doi.org/10.1016/j.neuint.2013.06.004.
X. Wei, B. Zhang, L. Cheng, et al., “Hydrogen sulfide induces neuroprotection against experimental stroke in rats by down-regulation of AQP4 via activating PKC,” Brain Res., 1622, 292–299 (2015). doi: https://doi.org/10.1016/j.brainres.2015.07.001.
Z. Jiang, C. Li, M. L. Manuel, et al., “Role of hydrogen sulfide in early blood-brain barrier disruption following transient focal cerebral ischemia,” PLoS One, 10, No. 2, e0117982 (2015). doi: https://doi.org/10.1371/journal.pone.0117982.
N. Ballatori, S. M. Krance, S. Notenboom, et al., “Glutathione dysregulation and the etiology and progression of human diseases,” Biol. Chem., 390, 3, 191–214 (2009). doi: https://doi.org/10.1515/BC.2009.033.
Y. Hu, R. Li, H. Yang, et al., “Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells,” J. Stroke Cerebrovasc. Dis., 24, No. 3, 601–609 (2015). doi: https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.10.006.
Q. Yu, Z. Lu, L. Tao, et al., “ROS-dependent neuroprotective effects of NaHS in ischemia brain injury involves the PARP/AIF pathway,” Cell. Physiol. Biochem., 36, No. 4, 1539–1551 (2015). doi: https://doi.org/10.1159/000430317.
A. K. Samhan-Arias, M. A. Garcia-Bereguiain, and C. Gutierrez-Merino, “Hydrogen sulfide is a reversible inhibitor of the NADH oxidase activity of synaptic plasma membranes,” Biochem. Biophys. Res. Commun., 388, No. 4, 718–722 (2009). doi: https://doi.org/10.1016/j.bbrc.2009.08.076.
M. Whiteman, J. S. Armstrong, S. H. Chu, et al., “The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ’scavenger’?,” J. Neurochem., 90, No. 3, 765–768 (2004). doi: https://doi.org/10.1111/j.1471-4159.2004.02617.x.
S. J. Chan, C. Chai, T. W. Lim, et al., “Cystathionine β-synthase inhibition is a potential therapeutic approach to treatment of ischemic injury,” ASN Neuro., 7, No. 2, 1759091415578711 (2015). doi: https://doi.org/10.1177/1759091415578711.
K. N. Islam, D. J. Polhemus, E. Donnarumma, et al., “Hydrogen sulfide levels and nuclear factor-erythroid 2-related factor 2 (NRF2) activity are attenuated in the setting of critical limb ischemia (CLI),” J. Am. Heart Assoc., 4 No. 5, e001986 (2015). doi: https://doi.org/10.1161/JAHA.115.001986.
R. J. Bridges, N. R. Natale, and S. A. Patel, “System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS,” Br. J. Pharmacol., 165, No. 1, 20–34 (2012). doi: https://doi.org/10.1111/j.1476-5381.2011.01480.x.
B. Adolf, P. Chapouton, C. S. Lam, et al., “Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon,” Dev. Biol., 29, No. 1, 278–293 (2006). doi: https://doi.org/10.1016/j.ydbio.2006.03.023.
Y. Ito, H. Tanaka, H. Okamoto, and T. Ohshima, “Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum,” Dev. Biol., 342, No. 1, 26–38 (2010). doi: https://doi.org/10.1016/j.ydbio.2010.03.008.
E. Than-Trong and L. Bally-Cuif, “Radial glia and neural progenitors in the adult zebrafish central nervous system,” Glia, 63, No. 8, 1406–1428 (2015). doi: https://doi.org/10.1002/glia.22856.
G. K. H. Zupanc and R. F. Sîrbulescu, “Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish,” Eur. J. Neurosci., 34, 917–929 (2011). doi: https://doi.org/10.1111/j.1460-9568.2011.07854.x.
E. V. Pushchina, A. A. Varaksin, D. K. Obukhov, and I. M. Prudnikov, “GFAP expression in the optic nerve and increased Н2S generation in the integration centers of the rainbow trout (Oncorhynchus mykiss) brain after unilateral eye injury,” Neural. Regen. Res., 15, No. 10, 1867–1886 (2020). doi:https://doi.org/10.4103/1673-5374.280320.
E. V. Pushchina, A. A. Varaksin, and D. K. Obukhov, “Cystathionine β-synthase in the brain of the trout Oncorhynchus mykiss after unilateral eye damage and in conditions of in vitro cultivation,” Russ. J. Dev. Biol., 50, 39–58 (2019).
E. V. Pushchina, A. A. Varaksin, and D. K. Obukhov, “Cystathionine β-synthase in the CNS of masu salmon Oncorhynchus masou (Salmonidae) and carp Cyprinus carpio (Cyprinidae),” Neurochem. J., 5, 24–34 (2011).
E. V. Pushchina and A. A. Varaksin, “Hydrogen sulfide, parvalbumin-, and GABA-producing system in the masu salmon brain,” Neurophysiology, 43, 109–122 (2011).
B. Cuoghi and L. Mola, “Macroglial cells of the teleost central nervous system: a survey of the main types,” Cell Tissue Res., 338, No. 3, 319–332 (2009).
E. V. Pushchina, S. Shukla, A. A. Varaksin, and D. K. Obukhov, “Cell proliferation and apoptosis in optic nerve and brain integration centers of adult trout Oncorhynchus mykiss after optic nerve injury,” Neural. Regen. Res., 11, No. 4, 578–590 (2016). doi: https://doi.org/10.4103/1673-5374.180742.
M. Arochena, R. Anadón, and S. M. Díaz-Regueira, “Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost,” J. Comp. Neurol., 469, No. 3, 413–436 (2004).
A. Alunni, S. Vaccari, S. Torcia, et al., “Characterization of glial fibrillary acidic protein and astroglial architecture in the brain of a continuously growing fish, the rainbow trout,” Eur. J. Histochem., 49, No. 2, 51–60 (2005).
M. Kálmán, “Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunohistochemical staining against glial fibrillary acidic protein (GFAP),” Anat. Embryol. (Berl.), 198, No. 5, 409–433 (1998).
J. Ganz, S. Kaslin, D. Hochmann, et al., “Heterogeneity and independence of adult neural progenitors in the zebrafish telencephalon,” Glia, 58, No. 11, 1345–1363 (2010). doi: https://doi.org/10.1002/glia.21012.
M. März, N. Chapouton, C. Diotel, et al., “Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon,” Glia, 58, No. 7, 870–888 (2010). doi: https://doi.org/10.1002/glia.20971.
E. V. Pushchina, A. A. Varaksin, and D. K. Obukhov, “Reparative neurogenesis in the brain and changes in the optic nerve of adult trout Oncorhynchus mykiss after mechanical damage of the eye,” Russ. J. Dev. Biol., 47, 11–32 (2016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pushchina, E.V., Marinina, K.S. & Myasoyedov, S.D. Hydrogen Sulfide and Pathophysiology of the CNS. Neurophysiology 52, 308–321 (2020). https://doi.org/10.1007/s11062-021-09887-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-021-09887-4