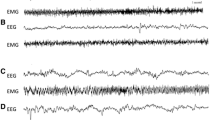
To induce an artificial hypometabolic state (AHMS) in rats and hamsters (non-hibernating and hibernating rodents, respectively), these animals were subjected to the complex action of hypoxia, hypercapnia, darkness, and low temperature. Natural winter hibernation was also induced in hamsters by housing them in a dark cold chamber. During recovering from the artificial and natural hypometabolic states, we recorded EEG activity (leads were implanted in the frontal and parietal cortices), EMG of the neck muscles, and body temperature. The initial period of self-heating after the AHMS in both species and that in hamsters after hibernation was characterized by low amplitudes of EEG and EMG and clearly pronounced depression of EEG oscillations of all frequency ranges, but with relative predominance of δ oscillations (the latter phenomenon may be partly due to superposition of ECG activity on EEG). In the course of further self-heating of animals, the EEG amplitude increased, and its spectral composition changed. The power of some EEG rhythms reached the maximum, and then the EEG composition was normalized successively beginning from the δ range; then the θ and α ranges and, finally, the βrange were normalized. We observed a certain parallelism between changes in the power of b activity in the composition of EEG and increase in the intensity of muscle activity. Patterns of EEG activity corresponding to one functional state or another (active or passive wakefulness, slow-wave sleep, or paradoxical sleep) were normalized in hamsters approximately two times faster than in rats.
Similar content being viewed by others
References
R. G. Melvin and M. T. Andrews, “Torpor induction in mammals: recent discoveries fuelling new ideas,” Trends Endocrinol. Metab., 20, 490-498 (2009).
H. V. Carey, M. T. Andrews, and S. L. Martin, “Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature,” Physiol. Rev., 83, No. 4, 1153-1181 (2003).
G. Heldmaier, S. Ortmann, and R. Elvert, “Natural hypometabolism during hibernation and daily torpor in mammals,” Respirat. Physiol. Neurobiol., 141, 317-329 (2004).
S. F. Morrison and K. Nakamura, “Central neural pathways for thermoregulation,” Front. Biosci., 16, 74-104 (2011).
K. L. Drew, C. L. Buck, B. M. Barnes, et al., “Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance,” J. Neurochem., 102, No. 6, 1713-1726 (2007).
Y. Tamura, M. Shintani, A. Nakamura, et al., “Phasespecific central regulatory systems of hibernation in Syrian hamsters,” Brain Res., 1045, 88-96 (2005).
H. R. Bouma, E. M. Verhaag, J. P. Otis, et al., “Induction of torpor: mimicking natural metabolic suppression for biomedical applications,” J. Cell. Physiol., 227, 1285-1290 (2012).
S. D. Mel’nichouk and D. O. Mel’nichouk, Hypobiosis of Animals (Molecular Mechanisms and Practical Importance for Agriculture and Medicine) [in Ukrainian], NAU, Kyiv (2007).
A. V. Shylo, V. V. Lomako, T. N. Bondar’, and G. A. Babiichouk, “Final metabolic products of nitric oxide under conditions of artificial hypometabolism in rats and hamsters,” Probl. Kriobiol., 15, No. 1, 3-13 (2005).
N. N. Timofeyev and L. P. Prokof’eva, Neurochemistry of Hypobiosis and Limits of Cryoresistance of the Organisms [in Russian], Meditsina, Moscow (1997).
D. A. Ignat’ev, R. Ya. Gordon, V. V. Vorob’iov, and V. V. Rogachevskii, “Comparative analysis of the processes of recovery of electroencephalographic and protein synthesis activity of the neocortex and hippocampus of winter-sleeping (susliks) and nonwinter-sleeping (rats) animals on coming out of hypothermia,” Biofizika, 50, No. 1, 140-151 (2005).
Ecological Physiology of Animals [in Russian], Part 1, A. A. Slonim (Ed.), Nauka, Leningrad (1979).
A. V. Shylo, V.V. Lomako, E. A. Ventsovskaya, and G. A. Babiichouk, “Bioelectrical activity of the rat brain and muscles on coming out of artificial hypometabolic state,” Probl. Kriobiol., 18, No. 3, 370-373 (2008).
M. B. Shtark, The Brain of Hibernators [in Russian], Nauka, Novosibirsk (1970).
A. V. Shylo, E. A. Ventsovskaya, and G. A. Babiichouk, “Change in the structure of sleep in rats after artificial hypometabolic state,” Probl. Kriobiol., 20, No. 1, 25-33 (2010).
H. Aihara, Y. Okada, and N. Tamaki, “The effects of cooling and rewarming on the neuronal activity of pyramidal neurons in guinea pig hippocampal slices,” Brain Res., 893, 36-45 (2001).
A. M. Ivanitsky, A. R. Nikolaev, and G. A. Ivanitsky, “Electroencephalography (Chapter 35),” in: Modern Techniques in Neuroscience Research, U. Windhorst and H. Johansson (eds.), Springer-Verlag, Berlin, Heidelberg (1999), pp. 971-996.
M. Steriade, P. Gloor, R. R. Llinas, et al., “Basic mechanisms of cerebral rhythmic activities,” Electroencephalogr. Clin. Neurophysiol., 76, 481-508 (1990).
M. Penttonen and G. Buzsáki, “Natural logarithmic relationship between brain oscillators,” Thalamus Relat. Syst., 2, 145-152 (2003).
P. Olejnicza, “Neurophysiologic basis of EEG,” J. Clin. Neurophysiol., 23, No. 3, 186-189 (2006).
J. E. Larkin and H. C. Heller, “The disappearing slow wave activity of hibernators,” Sleep Res. Online, 1, No. 2, 96-101 (1998).
J. H. Benington and H. C. Heller, “Restoration of brain energy metabolism as the function of sleep,” Prog. Neurobiol., 45, 347-360 (1995).
T. Porkka-Heiskanen, A. Kalinchuk, L. Alanko, et al., “Adenosine, energy metabolism, and sleep,” Sci. World J., 3, 790-798 (2003).
D. Ulrich and J. R. Huguenard, “Purinergic inhibition of GABA and glutamate release in the thalamus: implications for thalamic network activity,” Neuron, 15, 909-918 (1995).
T. R. Jinka, Ø. Tøien, and K. L. Drew, “Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A1 receptors,” J. Neurosci., 31, 10752-10758 (2011).
B. W. Iliff and S. J. Swoap, “Central adenosine receptor signalling is necessary for daily torpor in mice,” Am. J. Physiol. Regulat. Integrat. Comp. Physiol., 303, R477-R484 (2012).
D. Tupone, C. J. Madden, and S. F. Morrison, “Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat,” J. Neurosci., 33, No. 36, 14512-14525 (2013).
M. Muzzi, F. Blasi, A. Masi, et al., “Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia,” J. Cerebr. Blood Flow Metab., 33, 83-190 (2013).
V. M. Pickel, J. Chan, J. Linden, and D. L. Rosin, “Subcellular distributions of adenosine A1 and A2A receptors in the rat dorsomedial nucleus of the solitary tract at the level of the area postrema,” Synapse, 60, 496-509 (2006).
M. C. Andresen and D. L. Kunze, “Nucleus tractus solitarius - gateway to neural circulatory control,” Annu. Rev. Physiol., 56, 93-116 (1994).
W. H. Cao, C. J. Madden, and S. F. Morrison, “Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius,” Am. J. Physiol. Regulat. Integrat. Comp. Physiol., 299, R277-R290 (2010.
E. V. Golanov and D. J. Reis, “Neurons of nucleus of the solitary tract synchronize the EEG and elevate cerebral blood flow via a novel medullary area,” Brain Res., 892, 1-12 (2001).
S. Yan, A. Laferrière, C. Zhang, and I. R. Moss, “Microdialyzed adenosine in nucleus tractus solitarii and ventilatory response to hypoxia in piglets,” J. Appl. Physiol., 79, 405-410 (1995).
E. D. Martin, M. Fernández, G. Perea, et al., “Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission,” Glia, 55, 36-45 (2007).
S. Latini and F. Pedata, “Adenosine in the central nervous system: release mechanisms and extracellular concentrations,” J. Neurochem., 79, 463-484 (2001).
K. Plaschke, D. Boeckler, H. Schumacher, et al., “Adenosine-induced cardiac arrest and EEG changes in patients with thoracic aorta endovascular repair,” Br. J. Anaesthesia, 96, No. 3, 310-316 (2006).
G. Wassink, L. Bennet, J. O. Davidson, et al., “Preexisting hypoxia is associated with greater EEG activity during brief repeated asphyxia in near-term fetal sheep suppression and early onset of evolving seizure,” PLoS One, 8, No. 8, e73895 (2013).
C. G. von der Ohe, C. Darian-Smith, C. C. Garner, and H. C. Heller, “Ubiquitous and temperature-dependent neural plasticity in hibernators,” J. Neurosci., 26, No. 41, 10590-10598 (2006).
A. M. Magarinǒs, B. S. McEwen, M. Saboureau, and P. Pevet, “Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters,” Proc. Natl. Acad. Sci. USA, 103, No. 49, 18775-18780 (2006).
V. I. Popov and L. S. Bocharova, “Hibernation-induced structural changes in the synaptic contacts between mossy fibres and hippocampal pyramidal neurons,” Neuroscience, 48, 53-62 (1992).
V. I. Popov, L. S. Bocharova, and A. G. Bragin, “Repeated changes of dendritic morphology in the hippocampus of ground squirrels in the course of hibernation,” Neuroscience, 48, 45-51 (1992).
I. Bendtson, J. Gade, A. M. Rosenfalck, et al., “Nocturnal electroencephalogram registrations in type I (insulindependent) diabetic patients with hypoglycaemia,” Diabetologia, 34, 750-756 (1991).
W. D. Lust, A. B. Wheaton, G. Feussner, and J. Passonneau, “Metabolism in the hamster brain during hibernation and arousal,” Brain Res., 489, 12-20 (1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shylo, A.V. Dynamics of the Electrographic Indices in Rats and Hamsters Recovering from Artificial and Natural Hypometabolic States. Neurophysiology 47, 84–91 (2015). https://doi.org/10.1007/s11062-015-9502-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-015-9502-5