Abstract
Purpose
Post-operative MRI is used to assess extent of resection, monitor treatment response and detect progression in high-grade glioma. However, compliance with accepted guidelines for follow-up MRI, and impact on management/outcomes is unclear.
Methods
Multi-center, retrospective observational cohort study of patients with confirmed WHO grade 4 glioma (August 2018-February 2019) receiving oncological treatment. Primary objective: investigate follow-up MRI surveillance practice and compliance with recommendations from NICE (Post-operative scan < 72h, MRI every 3–6 months) and EANO (Post-operative scan < 48h, MRI every 3 months).
Results
There were 754 patients from 26 neuro-oncology centers with a median age of 63 years (IQR 54–70), yielding 10,100 (median, 12.5/person, IQR 5.2–19.4) person-months of follow-up. Of patients receiving debulking surgery, most patients had post-operative MRI within 72 h of surgery (78.0%, N = 407/522), and within 48 h of surgery (64.2%, N = 335/522). The median number of subsequent follow-up MRI scans was 1 (IQR 0–4). Compliance with NICE and EANO recommendations for follow-up MRI was 52.8% (N = 398/754) and 24.9% (N = 188/754), respectively. On multivariable Cox regression analysis, increased time spent in recommended follow-up according to NICE guidelines was associated with longer OS (HR 0.56, 95% CI 0.46–0.66, P < 0.001), but not PFS (HR 0.93, 95% CI 0.79–1.10, P = 0.349). Increased time spent in recommended follow-up according to EANO guidelines was associated with longer OS (HR 0.54, 95% CI 0.45–0.63, P < 0.001) but not PFS (HR 0.99, 95% CI 0.84–1.16, P = 0.874).
Conclusion
Regular surveillance follow-up for glioblastoma is associated with longer OS. Prospective trials are needed to determine whether regular or symptom-directed MRI influences outcomes.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most common malignant primary brain tumor [1], with a median overall survival (OS) of 12–15 months (range 6–17 months), even with maximal treatment involving surgical resection and chemoradiotherapy [2,3,4]. Magnetic Resonance Imaging (MRI) is used to assess extent of tumour resection, monitor treatment response and detect disease progression [5]. Expert bodies such as the National Institute of Health and Care Excellence (NICE) and European Association of Neuro-Oncology (EANO) offer consensus-based guidelines, for post-operative scheduled surveillance MRI [5, 6]. Clinical deterioration may occur between surveillance intervals and will often prompt an unscheduled MRI scan.
There is a lack of evidence supporting an optimal imaging strategy, which has been highlighted in a recent Cochrane review [7]. The James Lind Alliance (JLA) Neuro-Oncology Priority Setting Partnership (PSP) identified “what is the effect on prognosis of interval scanning to detect tumor recurrence, compared to scanning on symptomatic recurrence” as a research priority [8]. The National Cancer Research Institute (NCRI) Brain Tumor group emphasised the need for appropriately powered studies on imaging timing, to address this evidence gap [9]. One single-center study proposed an optimal MRI schedule for molecular subtypes of glioblastoma using a parametric model of progression free survival (PFS), for (IDH) wild-type GBM. They suggested scanning every 7.4 weeks until 120 weeks post standard treatment, followed by scans every 27.6 weeks following a 22-week inflection period [10]. Multiple international surveys have demonstrated wide variation in clinical practice [11, 12], and the impact of post-operative imaging schedules on management and outcomes is unclear [5, 13,14,15]. We conducted the ImagiNg Timing aftER glioblastoma surgery: an eVALuation of practice in Great Britain and Ireland (INTERVAL-GB) study to determine the MRI surveillance practice in the UK, and if adherence to NICE/EANO guidelines is associated with OS and PFS.
The primary objective was to assess nationwide follow-up imaging schedules and indications according to NICE and EANO guidelines, with secondary objectives assessing the association of follow-up MRI schedules on OS and PFS.
Methods
Study design, setting and participants
This was a multicenter, retrospective cohort study of patients with newly-diagnosed glioblastoma undergoing surgery and oncological therapy. The study was conducted in 26 neuro-oncology centers in the UK and Ireland. The study protocol has previously been published and provides a detailed overview of the study design [16,17,18]. The analysis plan was changed once after protocol publication; changing the definition of ‘compliance’ to imaging guidelines from a binary variable (Compliant and noncompliant) to a continuous variable (time spent compliant), to acknowledge that many patients spent periods of time as compliant, and noncompliant, during the follow-up period. Local audit and Caldicott guardian approval was obtained at each unit before data collection could commence.
Data was captured consecutively on adult patients (aged ≥ 18 years) with a new histopathological diagnosis of glioblastoma (according to the diagnostic criteria at the time of diagnosis – the 2016 World Health Organisation [WHO] Classification) [19], who underwent surgery and any active oncological treatment between August 31, 2018, and February 1, 2019. Data collection took place between November 11, 2021, and May 22, 2022. Collaborators identified eligible patients by searching historic Multidisciplinary Team Meetings (MDTMs), histopathological and/or surgical records locally against the inclusion and exclusion criteria (Supplementary Table 1).
Procedures
We used the Castor (Castor, NY, USA) online database to securely collect and store data. Data was collected by local investigators from a combination of the patient’s clinical, radiological and histopathological records. The variable domains consisted of baseline clinical and radiological variables, surgical and histopathological variables, adjuvant and supportive treatment details, follow-up MRI details and outcome measures. Follow-up MRI outcomes were determined by the overall scan report reported by a neuroradiologist at each participating center- and classified into three groups: stable, recurrence/further growth, and pseudoprogression. To be entered, reports had to be confirmed by MDTM agreement. The minimum data requirement for a case to be included was the baseline characteristics, surgery date, and post-operative follow-up details.
Outcomes
The primary outcome was compliance with follow-up MRI surveillance schedules defined in the latest NICE and EANO guidelines (see Supplementary Table 2 for definitions of compliance) [5, 6]. In summary, the NICE guidelines recommend imaging every three to six months post-surgery for two years, and EANO recommend a scheduled scan every three months for two years. If imaging was not completed prior to these dates, a patient was defined as being non-compliant, until the next scan was completed (correlating with time spent compliant to imaging recommendations). The secondary outcomes were OS and PFS. OS was defined as the date of surgery to date of death from any cause. PFS was defined as the time from date of surgery until MRI evidence of tumor recurrence, validated by MDTM agreement. Additional secondary outcomes included initiation of second-line chemotherapy, and re-intervention (repeat surgery or repeat radiotherapy). Follow-up was defined as until date of last clinic appointment, scan (MRI/CT), or date of death. Patients were censored from PFS if they progressed, or death occurred.
Sample size
The required sample size was derived following a three-center pilot study of 123 patients. The null hypothesis of the study was that more than half of patients (> 50%) will not be scanned in accordance with NICE Guidelines, and that groups scanned according to these guidelines will demonstrate improved OS (superiority assumption), and PFS. This identified a 34% proportion of patients compliant with NICE guidelines, and a median survival of 9.6 months. A hazard ratio of 1.35 was used to set the minimal clinically important difference for the study, which roughly equates to 3 months of OS benefit (similar survival benefit to Temozolomide in Stupp trial). Assuming a mortality rate of 85% of patients during the 24-month follow-up period, to achieve 80% power, with a 5% type 1 error rate, the sample size using a 2 sample, means superiority calculation was 456 patients (approximately 22 Neuro-Oncology units, assuming an average patient list of 20 patients per center).
Statistical analysis
Data analysis was carried out using R version 4.0.2 using the gpplot2, survminer, survival, and forcats, packages [20,21,22]. Compliance to NICE and EANO guidelines was summarised using descriptive statistics. This consisted of 3 groups- a group that had every follow-up scan within the recommended time period (Fully compliant); a group that had mixed periods of compliance with imaging, and mixed periods of non-compliance during follow-up (Inbetween); and a group that had no imaging carried out within the recommended timeframe (Never compliant). Fully compliant was further defined as: every scheduled follow-up scan occurring in equal or more frequently than the imaging recommendations (i.e. a patient having three scans, all of which occurred two months apart would be fully compliant). Inbetween was defined as at least one period of time where imaging occurred less frequently than recommended, alongside at least one period of recommended imaging (i.e. a patient who had three follow-up scans, the first two being three-six months apart, followed by a third scan nine months after last scan. Never compliant was defined as having no scheduled imaging within the recommended period (i.e. a patient who had two follow-up scans, both 9 months apart). In the event that a patient had no post-operative imaging, but had survived long enough to have imaging, they were defined as never compliant.
Unscheduled scans were not considered in the compliance definition- if an unscheduled scan occurred, the patient would remain compliant, and the time window for the next scan re-set, as if they had a scheduled scan (i.e. would have 3–6 months to have a further scheduled scan before being considered non-compliant). Continuous variables were analysed using mean (standard deviation [SD]), or median (interquartile range [IQR]) [23]. Differences in characteristics of compliant and non-compliant groups were presented with descriptive statistics. PFS and OS was estimated using Kaplan–Meier survival method. To identify if imaging frequency was associated with increased survival, and to account for any differences in characteristics associated with survival benefit, we conducted multivariable, Cox regression analysis, incorporating compliance as a variable alongside variables known to affect glioblastoma outcomes (age, performance status, extent of resection [EOR], and Stupp protocol treatments) [24,25,26,27,28,29]. As compliance is not a binary variable, and can change over time (i.e. a patient who is initially compliant with imaging guidelines for the first 12 months of follow-up before becoming non-compliant), compliance was classified as a time varying covariate, with periods of compliance a separate component [30]. This accounts for each individual period spent compliant and noncompliant to imaging, to account for unequal or differential periods of follow-up, minimising bias (i.e. a patient who has spent 20 months compliant and two months non-compliant would be reflected proportionally in analysis). Two separate cox regression models were completed, with compliance according to guideline (NICE and EANO) included separately as a variable in each model (to avoid collinearity). We used Hazard Ratios (HR) and a 95% confidence interval to measure effect size. For each analysis conducted, the number of patients with sufficient data entry was used, giving varying numbers for each analysis point. As biopsy groups have a different scanning recommendation, we carried out additional sensitivity analysis by removing the group, then repeating the analysis.
Results
Clinical characteristics
There were 818 patients identified from 26 out of 32 neuro-oncology centers in the UK and Ireland, of which 754 met the minimum data requirement (Table 1). The median number of patients per center was 33 (IQR 17–42) (Supplementary Table 3). Surgical and adjuvant treatments are summarised in Table 1, and detailed in Supplementary Table 4. The median follow-up time after surgery was 10.5 months (IQR 5.3–19.4 months). The total follow-up time was 10,100 (median, 12.5 months/person, IQR 5.2–19.4) person-months of follow-up.
Imaging follow-up and compliance
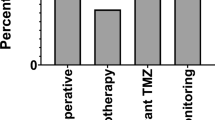
The post-surgical imaging schedules are summarised in Supplementary Table 5 and 6. In total, 522 (69.2%) patients had a post-operative MRI scan within 7 days of surgery. Of these, 462 (88.5%) were a post-operative scan to assess extent of resection, and 60 (11.5%) were for radiotherapy planning without an extent of resection scan. An MRI scan within the 72-h recommended NICE guidelines after resection (excluding biopsy) was seen in 78.0% (N = 407/522), and within the 48-h EANO guidelines in 64.2% (N = 335/522). The follow-up MRI scan timings after radiotherapy to assess for progression are presented in Fig. 1, and Supplementary Tables 5 and 6. At every follow-up scan, 40–50% showed disease progression. Pseudoprogression was seen in 8.3% of scans (median, IQR 7.8%-9.8%).
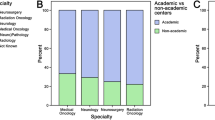
In total, 398 (52.8%) of patients had imaging intervals that were fully compliant with NICE guidelines, 70 (9.3%) had intervals that moved between recommended and noncompliant scanning, and 286 (37.9%) were never compliant. In total, for the EANO guidelines, 188 (24.9%) of patients were fully compliant; 217 (28.8%) moved between recommended and noncompliant scanning, and 349 (46.3%) were never compliant. Compliance differed depending on EOR, and Stupp protocol completion (Fig. 2, Supplementary Table 7).
Differences in interval imaging compliance stratified by extent of resection groups, Stupp protocol completion, and WHO performance status (PS). Fully compliant = a group that had every follow-up scan within the recommended time period; Inbetween = a group that had mixed periods of compliance with imaging, and mixed periods of non-compliance during follow-up; Never compliant = a group that had no imaging carried out within the recommended timeframe. STUPP protocol = full completion of STUPP protocol (adjuvant radiotherapy + concomitant and adjuvant chemotherapy); Other STUPP = Any other treatment (partial completion, no treatment)
Progression free survival and overall survival
In total, 684 (90.7%) progressed during the study period. The median PFS was 6.7 months (95% CI 6.2–7.3 months) (Supplementary Fig. 1). 641 patients (85.0%) died during the study period. The median OS using the KM estimate method was 11.4 months (95% CI 10.4–12.4 months), with expected differences depending on EOR, and STUPP protocol adherence (Supplementary Fig. 1). In total, of those that progressed radiologically, 305 (79.0%) had first progression detected using scheduled MRI, with 86 (21.0%) having progression first detected on unscheduled MRI. Of those who died, 305 (47.6%) had progression on MRI before death, with 336 (52.4%) without MRI confirmation of progression. Treatments for progression are shown in Supplementary Table 4. Patients with full and in between compliance were more likely to receive second-line chemotherapy, and re-operation (Supplementary Table 8). Survival differed between the three compliance groups (Fig. 3).
Association between MRI compliance and outcomes
The multivariable cox regression analysis results are shown in Table 2. On multivariable analysis including age, performance status, EOR, and completion of STUPP protocol, compliance with NICE recommendations (time varying covariate) was associated with increased OS (HR 0.56, 95% CI 0.46–0.66, P < 0.001). Compliance with EANO recommendations was also associated with increased OS (HR 0.54, 95% CI 0.45–0.63, P < 0.001). Compliance with NICE recommendations was not associated with increased PFS (HR 0.93, 95% CI 0.79–1.10, P = 0.349). Compliance with EANO recommendation was not associated with increased PFS (HR 0.99, 95% CI 0.84–1.16, P = 0.874). After repeated subgroup multivariable analysis excluding the biopsy group, provided similar results (Supplementary Table 9).
Discussion
In this study of patients with glioblastoma, only 52.8% were fully compliant with NICE recommended MRI follow-up schedules, and this was independently associated with an increase in OS compared to those who were not compliant. This study includes real-world data from 26 participating institutions across the UK and Ireland.
Patients in the regular scheduled MRI group had a survival benefit compared to the un-scheduled group. This finding is unexpected, and one possible explanation is that patients who underwent regular imaging had a better pre-operative performance status, were less likely to have a biopsy, and more likely to have completed additional, life-extending treatments [31, 32]. The hypothesis that scheduled MRI will detect asymptomatic disease progression prompting earlier initiation of second line treatments that can stabilise the glioblastoma and increase survival has been extensively debated [9, 33, 34], but remains untested. In our study, patients who had regular scheduled imaging had a higher likelihood of receiving second-line chemotherapy or re-operation, which could support this, but would need to be validated by studies to establish a clear temporal causality- a prospective study to test this hypothesis is unlikely. Reverse causality may affect the association between regular imaging schedule and survival, where patients with better survival are more likely to have regular imaging. Although OS and PFS were longer in the groups who received regular imaging, this could be due to a healthier cohort overall not captured by our baseline characteristics data points. No studies at present have examined this potential effect [35, 36]. Future studies investigating patient fitness in a more comprehensive manner, for example assessing daily activity including steps peri-operatively could provide us better insight on the matter. The finding that the non-full compliant group (Inbetween) had the largest OS by a significant margin, is surprising. This is most likely explained by reverse causality. By nature of having a prolonged follow-up period, due to inherently longer OS, these patients will have had more scans, and were therefore more likely to belong to this group, as opposed to the fully compliant group. Given the median number of post-operative follow-up scans was one, a patient with five or more scans would be more likely to survive longer, but belong to the inbetween category as opposed to the fully compliant group.
The most frequently performed MRI schedule was every 3 months (NICE recommended), although most patients only received one follow up scan in total (excluding the post-operative, and radiotherapy planning MRIs). It is recognised that glioblastoma is incurable for nearly all patients, and that disease progression is inevitable [3]. In our study, most progression occurred after the second follow-up scan after completing radiotherapy and concomitant temozolomide therapy, conducted at 6 months (52.8%). At every follow-up scan, 40–50% showed disease progression.
The patients in this study reflect a broad, less-selected, real-world representation of outcomes for glioblastoma in clinical practice, which explains the shorter OS compared to published trials (11.4 months versus 15–17 months in clinical trial cohorts) [37, 38]. Patients enrolled into clinical trials are more highly selected, often include patients who complete Stupp protocol treatments in full, which may over-estimate GBM survival [39]. Our study included patients who received a biopsy plus any adjuvant radiotherapy and chemotherapy regimes (e.g., short course radiotherapy), which are often excluded from trials, despite being a proportionally larger component of real-world glioblastoma cohorts [26, 40]. This may also explain the difference in pseudoprogression results in our study compared to trials [41].
A study of 277 high grade glioma patients (178 glioblastoma and 99 anaplastic astrocytoma) all treated with maximal surgery, radiotherapy, and temozolomide chemotherapy, proposed optimal timing for MRI monitoring to detect progression in glioblastoma monitoring as, every 7.4 weeks until the end of standard treatment, followed by a gap of 22 weeks, followed by every 27.6 weeks thereafter [10]. As our median time to progression was 6 months (24 weeks), this would mean most patients have progression identified during the inflection period, before the 27.6-week gap. A Cochrane review [7] identified that imaging studies are severely lacking. A single center study reported that having a post-operative MRI scan within 48 h found no association with improved survival- this did not include dynamic follow-up scanning [42]. The GIN-CUP study surveyed national imaging practice, identifying great variability across the UK [11]. MRI surveillance schedule recommendations also vary in international guidelines. For example, NICE recommends 3 monthly for the first 2 years [6], EANO recommends assessing EOR between 24 and 48 h of surgery, and an MRI scan 3–4 weeks after completion of radiotherapy, but a wide interval range of scans every 2–6 months ‘depending on the disease histology' and short-term MRI within 4–8 weeks to confirm disease progression [5]. USA guidelines do not make a formal recommendation for post-operative imaging intervals [5, 43], and instead advocate the incorporation of clinical trial guidelines into clinical practice [43], but these do not reference timing specifically. They do advocate the Response Assessment in Neuro-Oncology (RANO) criteria, but these do not make interval recommendations outside of a moratorium for expecting pseudoprogression [44]. This variation in guidance reflects the paucity of high-quality evidence and a reliance on expert evidence and consensus opinion.
This study has several strengths, and implications for policy. Namely, the study included patients from 26 Neuro-Oncology units and their associated neurosurgical centers in the United Kingdom and Ireland, including over 750 patients with a glioblastoma. This provides evidence from multiple centers, that employ different imaging strategies, increasing generalisability. Importantly, this study provides evidence that regular surveillance in-line with NICE guidance, was independently associated with increased OS, which has not been previously reported [33, 34].
Additionally, this study also describes the association between MRI and OS in a multi-center setting. The study comprises one of the largest glioblastoma cohorts, including data over 6 months from 85% of eligible neuro-oncology centers in the UK and Ireland, with a combined catchment population of 72 million people. The exact progression timeline of glioblastoma has been accurately mapped through this analysis, using the dates of each scan. This indicates that at every 3-month surveillance scan, there is a 40–50% chance of progression.
Furthermore, the results from this study address a key question from the James Lind Alliance Priority Setting Partnerships (PSP)—namely, the association of interval imaging on survival which previous literature has failed to address. The results are useful for guiding patients on likely scan schedules they may face, such as the time length between scans, and scan number. As such, the study findings can inform the development of both imaging studies and trials that investigate the use of imaging protocols on detection of, and initiation of treatments, but also all glioblastoma trials, looking to identify when progression occurs, and the impact of conventional treatments on survival. The implications for policy are that, given the association of regular imaging with improved OS and second-line treatment initiation, regular imaging recommendations should be considered as part of departmental policy/guidelines.
There are several study limitations. Firstly, this was a retrospective, observational study, and therefore does not provide high level evidence [45]. Secondly, the study is representative of UK and Ireland practice, and the findings may not be generalizable to other healthcare settings. Thirdly, there is no agreed definition of ‘compliant’, and the definition used does not permit for realistic unavoidable variation observed in every-day healthcare such as: no shows, waiting lists and machine maintenance. Fourthly, we excluded patients who only underwent biopsy with no adjuvant treatment, which constitutes around 20% of glioblastoma patients. Fifth, the number of records included in each analysis was not homogenous throughout, and there was substantial loss-to follow up data, particularly in adjuvant treatment details (15.8%). We did not correct for this, and for most comparisons, 85% of patients had data available for key outcomes analysed. The magnitude of this effect on our results is unclear.
Furthermore, there was no data retrieved to establish which scans were requested by clinicians but not attended due to patient choice or if too unwell to attend. As such there is a possibility that the irregularly scanned cohort were more likely to be too unwell to attend their appointment and thus, scanning was less frequent. This is supported by Fig. 2, which showcases that compliance declined with worsening pre-operative performance status. The GIN-CUP study highlighted that this could also be because of institutional policies, and that patients are often not scanned due to treating clinician preferences [11].
Finally, our progression definition was defined pragmatically by neuroradiologist interpretation, followed by validation at the neuro-oncology MDTM (tumor board). We did not use RANO defined definitions as these are not used routinely in clinical practice and are mainly used in clinical trials [44]. Therefore, there may be a lack of uniform definitions for pseudoprogression, recurrence, and stable disease in our study.
Conclusions
In this retrospective, multi-center study, we identified a variation in MRI timing after surgery for glioblastoma in the UK and Ireland. Adherence to MRI follow-up guidelines was low, but was associated with longer overall survival. Prospective studies are needed to investigate the impact of different MRI follow-up schedules, compared to standard practice and/or symptom-directed MRI for the detection of progression, treatment modalities, and overall survival benefit.
Data availability
Study data is available (on reasonable request) by contacting the corresponding authors.
References
Ostrom QT et al (2020) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol 22(12 Suppl 2):iv1–iv96
Brodbelt A et al (2015) Glioblastoma in England: 2007–2011. Eur J Cancer 51(4):533–542
Stupp R et al (2005) Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 352(10):987–996
Delgado-López PD, Corrales-García EM (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18(11):1062–1071
Weller M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186
NICE (2021) Brain tumours (primary) and brain metastases in over 16s. Available from: https://www.nice.org.uk/guidance/ng99/chapter/recommendations
Thompson G et al (2019) Interval brain imaging for adults with cerebral glioma. Cochrane Database Syst Rev 12(12):CD013137
JL A. Neuro-oncology Top 10. 2015; Available from: https://www.jla.nihr.ac.uk/priority-setting-partnerships/neuro-oncology/top-10-priorities/
Booth TC et al (2021) A position statement on the utility of interval imaging in standard of care brain tumour management: defining the evidence gap and opportunities for future research. Front Oncol 11:620070
Ji SY et al (2021) Radiological assessment schedule for high-grade glioma patients during the surveillance period using parametric modeling. Neuro Oncol 23(5):837–847
Booth TC et al (2021) Glioblastoma post-operative imaging in neuro-oncology: current UK practice (GIN CUP study). Eur Radiol 31(5):2933–2943
Thust SC et al (2018) Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur Radiol 28(8):3306–3317
Ellingson BM et al (2015) Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol 17(9):1188–1198
Kaufmann TJ et al (2020) Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro Oncol 22(6):757–772
Dumba M et al (2022) Imaging in patients with glioblastoma: a national cohort study. Neurooncol Pract 9(6):487–495
Gillespie CS et al (2022) Imaging timing after glioblastoma surgery (INTERVAL-GB): protocol for a UK and Ireland, multicentre retrospective cohort study. BMJ Open 12(9):e063043
Park JJ et al (2022) The Neurology and Neurosurgery Interest Group (NANSIG)-ten years of cultivating interest in clinical neurosciences. Acta Neurochir (Wien) 164(4):937–946
Gillespie CS et al (2021) Inspiring the next generation. Lancet Neurol 20(4):256–257
Louis DN et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820
ggplot (2009) Available from: https://citations.springernature.com/book?doi=https://doi.org/10.1007/978-0-387-98141-3
Survminer. Available from: https://cran.r-project.org/web/packages/survminer/index.html
forcats. Available from: https://cran.r-project.org/web/packages/forcats/index.html
Ghasemi A, Zahediasl S (2012) Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab 10(2):486–489
Molinaro AM et al (2020) Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol 6(4):495–503
Brown TJ et al (2016) Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol 2(11):1460–1469
Stupp R et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
SongTao Q et al (2012) IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 103(2):269–273
Hegi ME et al (2005) MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med 352(10):997–1003
Rivera AL et al (2010) MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol 12(2):116–121
Zhang Z et al (2018) Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 6(7):121
Tan AC et al (2020) Management of glioblastoma: State of the art and future directions. CA: A Cancer J Clin 70(4):299–312
Zur I et al (2020) Survival impact of the time gap between surgery and chemo-radiotherapy in Glioblastoma patients. Sci Rep 10(1):9595
Pasqualetti F et al (2022) Role of magnetic resonance imaging following postoperative radiotherapy in clinical decision-making of patients with high-grade glioma. Radiol Med (Torino) 127(7):803–808
Kraus RD et al (2022) Incidence and extent of disease progression on MRI between surgery and initiation of radiotherapy in glioblastoma patients. Neuro-Oncol Pract 9(5):380–389
Poon MTC et al (2020) Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep 10(1):11622
Eijgelaar RS et al (2018) Earliest radiological progression in glioblastoma by multidisciplinary consensus review. J Neurooncol 139(3):591–598
Gilbert MR et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Reardon DA et al (2020) Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol 6(7):1003–1010
Liu Y, Wasilewski A, Mohile NA (2020) Disparities in patient enrollment on glioblastoma clinical trials. CNS Oncol 9(2):Cns59
Stupp R et al (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48(14):2192–2202
Wick W et al (2016) Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol 18(10):1434–1441
Mrowczynski OD et al (2018) Utility of Early Postoperative Magnetic Resonance Imaging After Glioblastoma Resection: Implications on Patient Survival. World Neurosurg 120:e1171–e1174
Wen PY et al (2020) Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol 22(8):1073–1113
Wen PY et al (2017) Response Assessment in Neuro-Oncology Clinical Trials. J Clin Oncol 35(21):2439–2449
Durieux N, Vandenput S, Pasleau F (2013) OCEBM levels of evidence system. Rev Med Liege 68(12):644–649
Acknowledgements
The authors would like to thank the Neurology and Neurosurgery Interest Group (NANSIG), the British Neurosurgical Trainee Research Collaborative (BNTRC), the steering committee, all collaborators, and the Brain Tumour charity, without which this study would not have been possible
INTERVAL-GB Collaborative:
Conor S Gillespie, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Emily R Bligh, Department of Neurosurgery, Institute of Neurological Sciences (INS), Glasgow;
Michael TC Poon, Department of Neurosurgery, Western General Hospital, Edinburgh;
Abdurrahman I Islim, Department of Neurosurgery, Salford Royal, Manchester;
Georgios Solomou, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Melissa Gough,Department of Neurosurgery, Royal Victoria Infirmary, Newcastle;
Christopher P Millward, Department of Neurosurgery, The Walton Centre, Liverpool;
Ola Rominiyi, Department of Neurosurgery, Royal Hallamshire Hospital, Sheffield;
Rasheed Zakaria, Department of Neurosurgery, The Walton Centre, Liverpool;
Stephen J. Price, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Colin Watts, Department of Neurosurgery, University Hospitals Birmingham, Birmingham;
Sophie Camp, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Thomas C Booth, Department of Neuroradiology, King’s College London Hospital, London;
Gerard Thompson, Department of Neuroradiology, Western General Hospital, Edinburgh;
Samantha J Mills, Department of Neuroradiology, The Walton Centre, Liverpool;
Adam Waldman, Department of Neuroradiology, Western General Hospital, Edinburgh;
Paul M. Brennan, Department of Neurosurgery, Western General Hospital, Edinburgh;
Michael D Jenkinson, Department of Neurosurgery, The Walton Centre, Liverpool;
Hidayatul Abdullmalek, Department of Neurosurgery, James Cook University Hospital, Middlesbrough;
Suhaib Abualsaud, Department of Neurosurgery, Royal Hallamshire Hospital, Sheffield;
Gideon Adegboyega, Department of Neurosurgery, Queen’s Hospital, Romford, Essex;
Chinelo Afulukwe, Department of Neurosurgery- Southmead Hospital, Bristol;
Najma Ahmed, Department of Neurosurgery, King’s College London Hospital, London;
Michael Amoo, Department of Neurosurgery, Beaumont Hospital, Dublin;
Abdelsalam Nedal Al-Sousi, Department of Neurosurgery, Beaumont Hospital, Dublin;
Yahia Al-Tamimi, Department of Neurosurgery, Royal Hallamshire Hospital, Sheffield;
Ajitesh Anand, Department of Neurosurgery, Ninewells Hospital, Dundee;
Neil Barua, Department of Neurosurgery- Southmead Hospital, Bristol;
Harsh Bhatt, Department of Neurosurgery, University Hospital of Wales, Cardiff;
Ion Boiangiu, Department of Neurosurgery, University Hospitals Coventry, Coventry;
Abbey Boyle, Department of Neurosurgery, Leeds General Infirmary, Leeds;
Christiaan Bredell, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Talhah Chaudri, Department of Neurosurgery, University Hospitals Birmingham, Birmingham;
Jeremy Cheong, Department of Neurosurgery, Royal Victoria Infirmary, Newcastle;
Ana Cios, Department of Neurosurgery, Royal Victoria Hospital, Belfast;
David Coope, Department of Neurosurgery, Salford Royal, Manchester;
Ian Coulter, Department of Neurosurgery, Royal Victoria Infirmary, Newcastle;
Giles Critchley, Department of Neurosurgery, Royal Sussex County Hospital, Brighton;
Harriet Davis, Department of Neurosurgery, Institute of Neurological Sciences (INS), Glasgow;
Paolo Jose De Luna, Department of Neurosurgery, Royal London Hospital, London;
Nayan Dey, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Bea Duric, Department of Neurosurgery, King’s College London Hospital, London;
Abdullah Egiz, Department of Neurosurgery, Royal Preston Hospital, Preston;
Justyna O. Ekert, Victor Horsley Department of Neurosurgery, National Hospital for Neurology and Neurosurgery, London;
Chinedu Brian Egu, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Jinendra Ekanayake, Department of Neurosurgery, Royal Sussex County Hospital, Brighton;
Anna Elso, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Tomas Ferreira, Department of Neurosurgery- Southmead Hospital, Bristol;
Tom Flannery, Department of Neurosurgery, Royal Victoria Hospital, Belfast;
Kwan Wai Fung, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Rahul Ganguly, Department of Neurosurgery, St George’s Hospital, London;
Sanay Goyal, Department of Neurosurgery, University Hospital of Wales, Cardiff;
Emily Hardman, Department of Neurosurgery, Salford Royal, Manchester;
Lauren Harris, Department of Neurosurgery, Queen’s Hospital, Romford, Essex;
Theodore Hirst, Department of Neurosurgery, Royal Victoria Hospital, Belfast;
Kelvin Sunn Hoah, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Sam Hodgson, Department of Neurosurgery, University Hospitals Birmingham, Birmingham;
Kismet Hossain-Ibrahim, Department of Neurosurgery, Ninewells Hospital, Dundee;
Lena Mary Houlihan, Department of Neurosurgery, Beaumont Hospital, Dublin;
Sami Squali Houssaini, Department of Neurosurgery, Salford Royal, Manchester;
Sadid Hoque, Department of Neurosurgery, Queen’s Hospital, Romford, Essex;
Dana Hutton, Department of Neurosurgery, Ninewells Hospital, Dundee;
Mahnoor Javed, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Neeraj Kalra, Department of Neurosurgery, Leeds General Infirmary, Leeds;
Siddarth Kannan, Department of Neurosurgery, Royal Preston Hospital, Preston;
Efthymia Maria Kapasouri, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Andrew Keenlyside, Department of Neurosurgery, Ninewells Hospital, Dundee;
Kristy Kehoe, Department of Neurosurgery, Royal Victoria Infirmary, Newcastle;
Bharti Kewlani, Department of Neurosurgery, Beaumont Hospital, Dublin;
Prerna Khanna, Department of Neurosurgery, Western General Hospital, Edinburgh;
Rosaline de Koning, Department of Neurosurgery, John Radcliffe Hospital, Oxford;
Kunalika Sathish Kumar, Department of Neurosurgery, Beaumont Hospital, Dublin;
Ashvin Kuri, Department of Neurosurgery, Royal London Hospital, London;
Simon Lammy, Department of Neurosurgery, Institute of Neurological Sciences (INS), Glasgow;
Eunkyung Lee, Department of Neurosurgery, Derriford Hospital, Plymouth;
Robert Magouirk, Department of Neurosurgery, Ninewells Hospital, Dundee;
Andrew J Martin, Department of Neurosurgery, St George’s Hospital, London;
Riccardo Masina, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Ryan Mathew, Department of Neurosurgery, Leeds General Infirmary, Leeds;
Adele Mazzoleni, Department of Neurosurgery, Royal London Hospital, London;
Patrick McAleavey, Department of Neurosurgery, Royal Victoria Hospital, Belfast;
Gráinne McKenna, Department of Neurosurgery, Royal London Hospital, London;
Daniel McSweeney, Department of Neurosurgery, Cork University Hospital, Cork;
Saad Moughal, Department of Neurosurgery, Leeds General Infirmary, Leeds;
Mohammad Arish Mustafa, Department of Neurosurgery, The Walton Centre, Liverpool;
Engelbert Mthunzi, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Armin Nazari, Department of Neurosurgery, Ninewells Hospital, Dundee;
Trinh Ton Nu Ngoc, Department of Neurosurgery, Beaumont Hospital, Dublin;
Shiva Nischal, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Michael O’Sullivan, Department of Neurosurgery, Cork University Hospital, Cork;
Jay J Park, Department of Neurosurgery, Western General Hospital, Edinburgh;
Jonathan Pesic Smith, Department of Neurosurgery, James Cook University Hospital, Middlesbrough;
Peter Peterson, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Isaac Phang, Department of Neurosurgery, Royal Preston Hospital, Preston;
Puneet Plaha, Department of Neurosurgery, John Radcliffe Hospital, Oxford;
Shyam Pujara, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
George E Richardson, Department of Neurosurgery, The Walton Centre, Liverpool;
Marwa Saad, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Shinjan Sangal, Department of Neurosurgery, Queen’s Hospital, Romford, Essex;
Avani Shanbhag, Department of Neurosurgery- Southmead Hospital, Bristol;
Veekshith Shetty, Department of Neurosurgery, St George’s Hospital, London;
Natalie Simon, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Robert Spencer, Department of Neurosurgery, University Hospital of Wales, Cardiff;
Rosa Sun, Department of Neurosurgery, University Hospitals Birmingham, Birmingham;
Irtiza Syed, Department of Neurosurgery, University Hospitals Coventry, Coventry;
Jesvin Tom Sunny, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Anca-Mihaela Vasilica, Department of Neurosurgery, National Hospital for Neurology and Neurosurgery, London;
Daniel O’Flaherty, Department of Neurosurgery, Royal Sussex County Hospital, Brighton;
Arslan Raja, Department of Neurosurgery, Western General Hospital, Edinburgh;
Daniele Ramsay, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Renitha Reddi, Department of Neurosurgery, Beaumont Hospital, Dublin;
Elena Roman, Department of Neurosurgery, Beaumont Hospital, Dublin;
Ola Rominiyi, Department of Neurosurgery, Royal Hallamshire Hospital, Sheffield;
Dorina Roy, Department of Neurosurgery, Royal Victoria Hospital, Belfast;
Omar Salim, Department of Neurosurgery, Institute of Neurological Sciences (INS), Glasgow;
Jeremiah Samkutty, Department of Neurosurgery, Royal Sussex County Hospital, Brighton;
Jashan Selvakumar, Department of Neurosurgery, St George’s Hospital, London;
Thomas Santarius, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Avani Shanbhag, Department of Neurosurgery- Southmead Hospital, Bristol;
Stuart Smith, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Agbolahan Sofela, Department of Neurosurgery, Derriford Hospital, Plymouth;
Edward Jerome St. George, Department of Neurosurgery, Institute of Neurological Sciences (INS), Glasgow;
Preethi Subramanian, Department of Neurosurgery, Royal London Hospital, London;
Vaibhav Sundaresan, Department of Neurosurgery, University Hospitals Coventry, Coventry;
Kieron Sweeney, Department of Neurosurgery, Beaumont Hospital, Dublin;
Boon Hoe Tan, Department of Neurosurgery, St George’s Hospital, London;
Nicole Turnbull, Department of Neurosurgery, University Hospitals Coventry and Warwickshire, Coventry;
Yuewei Tao, Department of Neurosurgery, Ninewells Hospital, Dundee;
Lewis Thorne, Victor Horsley Department of Neurosurgery, National Hospital for Neurology and Neurosurgery, London;
Rebecca Tweedie, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Anastasia Tzatzidou, Department of Neurosurgery, James Cook University Hospital, Middlesbrough;
Babar Vaqas, Department of Neurosurgery, Queen’s Hospital, Romford, Essex;
Sara Venturini, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Kathrin Whitehouse, Department of Neurosurgery, University Hospital of Wales, Cardiff;
Peter Whitfield, Department of Neurosurgery, Derriford Hospital, Plymouth;
Jack Wildman, Department of Neurosurgery- Southmead Hospital, Bristol;
Isabelle Williams, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Karl Williams, Department of Neurosurgery, University Hospitals Birmingham, Birmingham;
Victoria Wykes, Department of Neurosurgery, University Hospitals Birmingham, Birmingham;
Tiffany Tze Shan Ye, Department of Neurosurgery, University Hospital of Wales, Cardiff;
Kelvin Sunn Yap, Department of Neurosurgery, Imperial College Healthcare NHS Trust, London;
Mahir Yousuff, Department of Neurosurgery, Queen’s Medical Centre, Nottingham;
Asaad Zulfiqar, Department of Neurosurgery, Beaumont Hospital, Dublin;
Neurology and Neurosurgery Interest Group (NANSIG):
Soham Bandyopadhyay, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Setthasorn ZY Ooi, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Abigail Clynch, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Oliver Burton, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Moritz Steinruecke, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
William Bolton, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Alvaro Yanez Touzet, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Hannah Redpath, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Seong Hoon Lee, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Abdullah Egiz, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Joshua Erhabor, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Orla Mantle, The Neurology and Neurosurgery Interest Group (NANSIG), London, UK;
Conor S Gillespie, Department of Neurosurgery, Addenbrooke’s Hospital, Cambridge;
Emily R Bligh, Department of Neurosurgery, Institute of Neurological Sciences (INS), Glasgow;
British Neurosurgical Trainee Research Collaborative (BNTRC):
Angelos Kolias, British Neurosurgical Trainee Research Collaborative (BNTRC), London, UK;
Julie Woodfield, British Neurosurgical Trainee Research Collaborative (BNTRC), London, UK;
Aswin Chari, British Neurosurgical Trainee Research Collaborative (BNTRC), London, UK;
Robin Borchert, British Neurosurgical Trainee Research Collaborative (BNTRC), London, UK;
Rory Piper, British Neurosurgical Trainee Research Collaborative (BNTRC), London, UK;
Daniel M Fountain, British Neurosurgical Trainee Research Collaborative (BNTRC), London, UK;
Michael TC Poon, Department of Neurosurgery, Western General Hospital, Edinburgh;
Abdurrahman I Islim, Department of Neurosurgery, Salford Royal, Manchester;
Funding
The authors declare a research bursary from the North West Cancer Fund, used to pay for the Data collection tool, Castor, for 6 months use (award number N/A). TCB declares funding from a Wellcome Trust/ESPRC grant (award number WT203148/Z/16/Z).
Author information
Authors and Affiliations
Consortia
Contributions
Author contributions are detailed in Supplementary material. CSG, ERB, GS, AII, MTCP and MDJ conceived the study. GS, MG, CPM, OR, RZ, PB, SJP, CW, SC, TCB, GT, SJM, AW, and MDJ formed the INTERVAL-GB steering committee and provided senior support in study design, data collection, study results, and reviewed the draft manuscript. PB and MDJ are senior authors.
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Walton Centre NHS Foundation Trust, approval number NS 370. Each participating center obtained individual institutional approval before partaking in the study. As this was non-identifiable data, patient consent was not required. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
N/A.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
INTERVAL-GB Collaborative., Neurology and Neurosurgery Interest Group (NANSIG). & British Neurosurgical Trainee Research Collaborative (BNTRC). Imaging timing after surgery for glioblastoma: an evaluation of practice in Great Britain and Ireland (INTERVAL-GB)- a multi-centre, cohort study. J Neurooncol 169, 517–529 (2024). https://doi.org/10.1007/s11060-024-04705-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04705-3