Abstract
Purpose
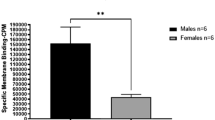
Iron plays a crucial role in various biological mechanisms and has been found to promote tumor growth. Recent research has shown that the H-ferritin (FTH1) protein, traditionally recognized as an essential iron storage protein, can transport iron to GBM cancer stem cells, reducing their invasion activity. Moreover, the binding of extracellular FTH1 to human GBM tissues, and brain iron delivery in general, has been found to have a sex bias. These observations raise questions, addressed in this study, about whether H-ferritin levels extrinsic to the tumor can affect tumor cell pathways and if this impact is sex-specific.
Methods
To interrogate the role of systemic H-ferritin in GBM we introduce a mouse model in which H-ferritin levels are genetically manipulated. Mice that were genetically manipulated to be heterozygous for H-ferritin (Fth1+/-) gene expression were orthotopically implanted with a mouse GBM cell line (GL261). Littermate Fth1 +/+ mice were used as controls. The animals were evaluated for survival and the tumors were subjected to RNA sequencing protocols. We analyzed the resulting data utilizing the murine Microenvironment Cell Population (mMCP) method for in silico immune deconvolution. mMCP analysis estimates the abundance of tissue infiltrating immune and stromal populations based on cell-specific gene expression signatures.
Results
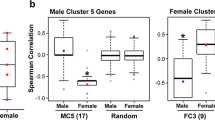
There was a clear sex bias in survival. Female Fth1+/- mice had significantly poorer survival than control females (Fth1+/+). The Fth1 genetic status did not affect survival in males. The mMCP analysis revealed a significant reduction in T cells and CD8 + T cell infiltration in the tumors of females with Fth1+/- background as compared to the Fth1+/+. Mast and fibroblast cell infiltration was increased in females and males with Fth1+/- background, respectively, compared to Fth1+/+ mice.
Conclusion
Genetic manipulation of Fth1 which leads to reduced systemic levels of FTH1 protein had a sexually dimorphic impact on survival. Fth1 heterozygosity significantly worsened survival in females but did not affect survival in male GBMs. Furthermore, the genetic manipulation of Fth1 significantly affected tumor infiltration of T-cells, CD8 + T cells, fibroblasts, and mast cells in a sexually dimorphic manner. These results demonstrate a role for FTH1 and presumably iron status in establishing the tumor cellular landscape that ultimately impacts survival and further reveals a sex bias that may inform the population studies showing a sex effect on the prevalence of brain tumors.












Similar content being viewed by others
Data availability
All data and methodology supporting the findings of this study are available within the paper. The RNA sequencing data is deposited at NCBI Gene Expression Omnibus repository.
References
Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D et al (2022) Epidemiology of glioblastoma multiforme–literature review. Cancers (Basel). 14(10). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9139611/
Tamimi AF, Juweid M (2017) Epidemiology and outcome of Glioblastoma. Glioblastoma: 143–153. doi: https://www.ncbi.nlm.nih.gov/books/NBK470003/
McLendon RE, Halperin EC (2003) Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 98(8):1745–1748. https://doi.org/10.1002/cncr.11666
Lopes-Ramos CM, Quackenbush J, DeMeo DL (2020) Genome-wide sex and gender differences in Cancer. Front Oncol 10:2486. https://www.frontiersin.org/articles/. https://doi.org/10.3389/fonc.2020.597788/full
Khan MT, Prajapati B, Lakhina S, Sharma M, Prajapati S, Chosdol K et al (2021) Identification of gender-specific molecular differences in Glioblastoma (GBM) and Low-Grade Glioma (LGG) by the analysis of large transcriptomic and epigenomic datasets. Front Oncol 11:3634. https://doi.org/10.3389/fonc.2021.699594
Franceschi E, Tosoni A, Minichillo S, Depenni R, Paccapelo A, Bartolini S et al (2018) The prognostic roles of gender and O6-Methylguanine-DNA methyltransferase methylation status in Glioblastoma Patients: the female power. World Neurosurg 112:e342–e347. https://pubmed.ncbi.nlm.nih.gov/29337169/
Whitmire P, Rickertsen CR, Hawkins-Daarud A, Carrasco E, Lorence J, Leon G, De et al (2018) Sex-specific impact of patterns of imageable tumor growth on survival of primary glioblastoma patients. https://doi.org/10.1101/325464v2. bioRxiv.:325464. https://www.biorxiv.org/content/
Yang W, Warrington NM, Taylor SJ, Whitmire P, Carrasco E, Singleton KW et al (2019) Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data [Internet]. Vol. 11, Sci. Transl. Med. 2019. doi: https://stm.sciencemag.org/
Ma J, Yao Y, Tian Y, Chen K, Liu B (2022) Advances in sex disparities for cancer immunotherapy: unveiling the dilemma of Yin and Yang. Biol Sex Differ 2022 131(1):1–12. https://bsd.biomedcentral.com/articles/https://doi.org/10.1186/s13293-022-00469-5
Irelli A, Sirufo MM, D’Ugo C, Ginaldi L, De Martinis M (2020) Sex and gender influences on cancer immunotherapy response. Biomedicines 8(7). https://doi.org/10.3390/biomedicines8070232
Han J, Yang Y, Li X, Wu J, Sheng Y, Qiu J et al (2022) Pan-cancer analysis reveals sex-specific signatures in the tumor microenvironment. Mol Oncol 16(11):2153–2173. https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/1878-0261.13203
Mackenzie EL, Iwasaki K, Tsuji Y (2008) Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid Redox Signal 10(6):997. pmc/articles/PMC2932529/
Chen Y, Fan Z, Yang Y, Gu C (2019) Iron metabolism and its contribution to cancer (review). Int J Oncol 54(4):1143–1154. http://www.spandidos-publications.com/https://doi.org/10.3892/ijo.2019.4720/abstract
Schonberg DL, Miller TE, Wu Q, Flavahan WA, Das NK, Hale JS et al (2015) Preferential Iron trafficking characterizes glioblastoma stem-like cells. Cancer Cell 28(4):441–455, https://doi.org/10.1016/j.ccell.2015.09.002
Brown RAM, Richardson KL, Kabir TD, Trinder D, Ganss R, Leedman PJ (2020) Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front Oncol 10:476. https://doi.org/10.3389/fonc.2020.00476
Basuli D, Tesfay L, Deng Z, Paul B, Yamamoto Y, Ning G et al (2017) Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 2017 3629 36(29):4089–4099. https://www.nature.com/articles/onc201711
O’Donnell KA, Yu D, Zeller KI, Kim J, Racke F, Thomas-Tikhonenko A et al (2006) Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Mol Cell Biol 26(6):2373. https://doi.org/10.1128/MCB.26.6.2373-2386.2006
Blight GD, Morgan EH (1983) Ferritin and iron uptake by reticulocytes. Br J Haematol 55(1):59–71. https://doi.org/10.1111/j.1365-2141.1983.tb01224.x
Shesh B, Slagle-Webb B, Shenoy G, Khristov V, Zacharia BE, Connor JR (eds) (2023) Uptake of H-ferritin by Glioblastoma stem cells and its impact on their invasion capacity. J Cancer Res Clin Oncol. https://pubmed.ncbi.nlm.nih.gov/37237166/
Sacco A, Battaglia AM, Botta C, Aversa I, Mancuso S, Costanzo F et al (2021) Iron metabolism in the tumor microenvironment—implications for anti-cancer immune response. Cells 10(2):1–17. https://doi.org/10.3390/cells10020303
Ferreira C, Santambrogio P, Martin ME, Andrieu V, Feldmann G, Hénin D et al (2001) H ferritin knockout mice: a model of hyperferritinemia in the absence of iron overload. Blood 98(3):525–532. https://ashpublications.org/blood/article/98/3/525/53241/H-ferritin-knockout-mice-a-model-of
Thompson K, Menzies S, Muckenthaler M, Torti FM, Wood T, Torti SV et al (2003) Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J Neurosci Res 71(1):46–63. https://www.researchgate.net/publication/10993145_Mouse_brains_deficient_in_H-ferritin_have_normal_iron_concentration_but_a_protein_profile_of_iron_deficiency_and_increased_evidence_of_oxidative_stress
Troike KM, Silver DJ, Ghosh PK, Mulkearns-Hubert EE, Hubert CG, Connor JR et al (2022) Tumor cell-intrinsic HFE drives glioblastoma growth. bioRxiv.:2022.04.13.487917. https://doi.org/10.1101/2022.04.13.487917v1. https://www.biorxiv.org/content/
Biskup E, Schejbel L, de Oliveira DNP, Høgdall E (2022) Test of the FlashFREEZE unit in tissue samples freezing for biobanking purposes. Cell Tissue Bank.:1–13. https://link.springer.com/article/10.1007/s10561-022-10045-1
Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F et al (2016) Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 17(1):1–20. https://genomebiology.biomedcentral.com/articles/https://doi.org/10.1186/s13059-016-1070-5
Petitprez F, Levy S, Sun CM, Meylan M, Linhard C, Becht E et al (2020) The murine Microenvironment Cell Population counter method to estimate abundance of tissue-infiltrating immune and stromal cell populations in murine samples using gene expression. Genome Med 12(1):1–15. https://genomemedicine.biomedcentral.com/articles/https://doi.org/10.1186/s13073-020-00783-w
Risso D, Ngai J, Speed TP, Dudoit S (2014) Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol 2014 329(9):896–902. https://www.nature.com/articles/nbt.2931
Ho KH, Patrizi A (2021) Assessment of common housekeeping genes as reference for gene expression studies using RT-qPCR in mouse choroid plexus. Sci Rep 11(1):3278. https://doi.org/10.1038/s41598-021-82800-5
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139. https://doi.org/10.1093/bioinformatics/btp616
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J et al (2003) PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003 343(3):267–273. https://www.nature.com/articles/ng1180
Rahnenfuhrer AA (2022) Bioconductor - topGO [Internet]. Bioconductor. 2022. p. 1. https://bioconductor.org/packages/release/bioc/html/topGO.html
Marisetty A, Wei J, Kong LY, Ott M, Fang D, Sabbagh A et al (2020) MiR-181 family modulates osteopontin in glioblastoma multiforme. Cancers 2020, Vol 12, Page 3813. 12(12):3813. doi: https://www.mdpi.com/2072-6694/12/12/3813/htm
Wen X, Li S, Guo M, Liao H, Chen Y, Kuang X et al (2020) miR-181a-5p inhibits the proliferation and invasion of drug-resistant glioblastoma cells by targeting F-box protein 11 expression. Oncol Lett 20(5). DOI: 10.3892/ol.2020.12098
Lv Z, Yang L (2013) MiR-124 inhibits the growth of glioblastoma through the downregulation of SOS1. Mol Med Rep 8(2):345–349. https://pubmed.ncbi.nlm.nih.gov/23817964/
Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M et al (2008) miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6(1):1–17. https://bmcmedicine.biomedcentral.com/articles/https://doi.org/10.1186/1741-7015-6-14
Mauldin IS, Jo J, Wages NA, Yogendran LV, Mahmutovic A, Young SJ et al (2021) Proliferating CD8 + T cell infiltrates are associated with improved survival in glioblastoma. Cells 10(12):3378. https://doi.org/10.3390/cells10123378
Vanoaica L, Richman L, Jaworski M, Darshan D, Luther SA, Kühn LC (2014) Conditional deletion of ferritin H in mice reduces B and T lymphocyte populations. PLoS ONE 9(2):e89270. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0089270
Vickman RE, Faget DV, Beachy P, Beebe D, Bhowmick NA, Cukierman E et al (2020) Deconstructing tumor heterogeneity: the stromal perspective. Oncotarget 11(40):3621. https://doi.org/10.18632/oncotarget.27736
Khalafallah AM, Huq S, Jimenez AE, Serra R, Bettegowda C, Mukherjee D (2021) Zooming in” on glioblastoma: Understanding tumor heterogeneity and its clinical implications in the era of single-cell ribonucleic acid sequencing. Neurosurgery 88(3):477–486. https://doi.org/10.1093/neuros/nyaa305
Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N et al (2010) Mast cells have a protumorigenic role in human thyroid cancer. Oncogene 29(47):6203–6215. https://pubmed.ncbi.nlm.nih.gov/20729915/
Johansson A, Rudolfsson S, Hammarsten P, Halin S, Pietras K, Jones J et al (2010) Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am J Pathol 177(2):1031–1041. https://pubmed.ncbi.nlm.nih.gov/20616342/
Põlajeva J, Sjösten AM, Lager N, Kastemar M, Waern I, Alafuzoff I et al (2011) Mast cell accumulation in glioblastoma with a potential role for stem cell factor and chemokine CXCL12. PLoS ONE 6(9). https://doi.org/10.1371/journal.pone.0025222
Ribatti D, Tamma R, Annese T (2020) Epithelial-mesenchymal transition in Cancer: A historical overview. Transl Oncol 13(6):100773. https://doi.org/10.1016/j.tranon.2020.100773
Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P (2005) Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol 23(35):8959–8967. https://pubmed.ncbi.nlm.nih.gov/16219934/
Ribatti D, Crivellato E (2009) The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol 275(C):89–131. https://pubmed.ncbi.nlm.nih.gov/19491054/
Seidel H, Hertfelder HJ, Oldenburg J, Kruppenbacher JP, Afrin LB, Molderings GJ (2021) Effects of primary mast cell disease on hemostasis and erythropoiesis. Int J Mol Sci 22(16). https://doi.org/10.3390/ijms22168960
Asif PJ, Longobardi C, Hahne M, Medema JP (2021) The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers (Basel). 13(18). https://doi.org/10.3390/cancers13184720
Song H, Fu X, Wu C, Li S (2020) Aging-related tumor associated fibroblasts changes could worsen the prognosis of GBM patients. Cancer Cell Int 20(1). https://doi.org/10.1186/s12935-020-01571-7
Ferreira C, Santambrogio P, Martin ME, Andrieu V, Feldmann G, Hénin D et al (2001) H ferritin knockout mice: a model of hyperferritinemia in the absence of iron overload. Blood 98(3):525–532. https://ashpublications.org/blood/article-pdf/98/3/525/1675612/h8150100525.pdf
Wu T, Li Y, Liu B, Zhang S, Wu L, Zhu X et al (2016) Expression of Ferritin Light Chain (FTL) is elevated in Glioblastoma, and FTL silencing inhibits Glioblastoma Cell Proliferation via the GADD45/JNK pathway. PLoS ONE 11(2):e0149361. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0149361
Rosager AM, Sørensen MD, Rikke H, Dahlrot DL, Schonberg, Jeremy N, Rich, Justin D, Lathia BWK (2017) Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: Expression and prognostic value. PLoS One. https://doi.org/10.1371/journal.pone.0182954
Acknowledgements
We sincerely thank Penn State College of Medicine’s Genomic Sciences Core members, Dr. Sirisha Pochareddy and Oana Bolt, for their assistance in tissue RNA isolation and mRNA sequencing-related efforts.
Funding
This work was funded by grant P01CA245705 by the National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
Conceptualization: Bhavyata (Pandya) Shesh, James R. Connor. Methodology: Bhavyata (Pandya) Shesh, Becky Slagle- Webb, Elizabeth Neely, Vonn Walter, Kondaiah Palsa, Todd Schell. Formal analysis and investigation: Bhavyata (Pandya) Shesh, Vonn Walter. Writing - original draft preparation: Bhavyata (Pandya) Shesh. Writing - review and editing: Bhavyata (Pandya) Shesh, James R. Connor, Vonn Walter. Funding acquisition: James R. Connor. Resources: James R. Connor, Vonn Walter, Todd Schell. Supervision: James R. Connor.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Mice used in this study were handled following an Institutional Animal Care and Use Committee (IACUC) - compliant protocol. Penn State Hershey College of Medicine IACUC # PROTO202001393 approved.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandya Shesh, B., Walter, V., Palsa, K. et al. Sexually dimorphic effect of H-ferritin genetic manipulation on survival and tumor microenvironment in a mouse model of glioblastoma. J Neurooncol 164, 569–586 (2023). https://doi.org/10.1007/s11060-023-04415-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04415-2