Abstract
Purpose
The Ki-67/MIB-1 labeling index (LI) is clinically used to differentiate between high and low-grade gliomas, while its prognostic value remains questionable. Glioblastoma (GBM) expressing wild-type isocitrate dehydrogenase IDHwt, a relatively common malignant brain tumor in adults, is characterized by a dismal prognosis. Herein, we have retrospectively investigated the prognostic role of Ki-67/MIB-1-LI in a large group of IDHwt GBM.
Methods
One hundred nineteen IDHwt GBM patients treated with surgery followed by Stupp’s protocol in our Institution between January 2016 and December 2021 were selected. A cut-off value for Ki-67/MIB-1-LI was used with minimal p-value based approach.
Results
A multivariate analysis showed that Ki-67/MIB-1-LI expression < 15% significantly correlated with a longer overall survival (OS), independently from the age of the patients, Karnofsky performance status scale, extent of surgery and O6-methylguanine (O6-MeG)-DNA methyltransferase promoter methylation status.
Conclusions
Among other studies focused on Ki-67/MIB-1-LI, this is the first observational study showing a positive correlation between OS of IDHwt GBM patients and Ki-67/MIB-1-LI that we propose as a new predictive marker in this subtype of GBM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM), the most common malignant central nervous system (CNS) tumor, is characterized by a short overall survival (OS). The standard of treatment for a GBM is surgery, followed by daily radiotherapy (RT) combined with temozolomide (TMZ), then followed by 6 cycles of TMZ [1, 2]. The use of biomarkers predicting prognosis and response to treatment are integrative parts of medical management in GBM patients. In this regard, the methylation status of the gene coding promoter for O6-methylguanine-DNA methyltransferase (MGMT) enzyme has been positively correlated with a prolonged survival in patients treated with TMZ-based therapy [3].
The new classification of tumors of CNS recently introduced, identifies a critical role for the mutation status of the isocitrate dehydrogenase gene (IDH) [4]. The expression of wildtype IDH (IDHwt), occurring in ∼90% of all GBM cases, results in a worse prognosis [5], whilst is a weak predictor of long-term survival in GBM patients [6]. Ki-67, a nuclear protein persistently expressed in all phases of the cell cycle, is widely used as a proliferation marker for human tumor cells [7]. MIB-1 is a monoclonal antibody that identifies the Ki-67 protein in paraffin tissue [8]. The Ki-67/MIB-1 labeling index (LI) is one of the immunohistochemical markers used for discriminating between high and low-grade gliomas [9], whilst its use as prognostic factor for the stratification is still discussed [10,11,12,13,14,15,16,17,18,19,20]. Thus, whilst some studies show no association between Ki-67 LI and survival [13, 14, 21], and no predictive value [10, 21], others show a poorer survival rate for lower Ki-67 LI [14, 19], whilst others the opposite [11, 12, 15,16,17,18, 20]. Notably, nowadays, no studies have still been performed on the relationship between Ki-67/MIB-1 labeling index and IDH1-WT status in GBM.
In the present study, we have investigated the power of Ki-67/MIB-1 expression as prognosticator in a large and homogenous group of patients suffering from IDH wild-type Glioblastoma (IDHwt GBM) prognostic impact of the Ki-67/MIB-1 labeling index.
Patients and methods
Patient characteristics
Between February 2016 and July 2021, 183 consecutive patients with GBM were treated at University Hospital of Siena, Italy. The main clinical data (extent of surgery, clinical examination, blood counts and chemistry, Karnofsky Performance Status – KPS) were registered in all patients. All GBMs were surgically removed and characterized for the MGMT methylation-, IDH1 mutation status, and Ki-67/MIB-1-LI score. One hundred and nineteen patients, characterized for MGMT status and IDH-wild type and MIB-1/Ki 67 labeling index are selected for the present analysis. Characteristics of patients are listed in Table 1. All patients received RT plus concomitant daily TMZ, followed by adjuvant TMZ. RT started within 6 weeks of surgery and consisted of fractionated focal irradiation, at the dose of 60/59.4 Gy in 30/33 fractions of 2/1.8 Gy each. Concomitant chemotherapy consisted of TMZ at the dose of 75 mg/m2, given 7 days per week from the first day of RT. Adjuvant TMZ was started 4 weeks after the end of RT and delivered for 5 days every 28 days up to 12 cycles. The dose was 150 mg/m2 for the first cycle and was increased to 200 mg/m2 from the second cycle. The dose was reduced or suspended in patients with disease progression or toxicity. MRI was repeated before RT, before the first cycle of adjuvant TMZ, and thereafter every 8 weeks or as appropriate according to neurological status. Neuroradiographic response was assessed by RANO criteria [22]. Tumor progression was defined by an increase in tumor size more than 25% or by the presence of a new lesion on imaging. Radiological progression had to be confirmed at two different MRI evaluations (at least 2 months apart). In patients with tumor progression, the recurrence was recorded at the time of the first MRI showing progression.
Treatment planning and treatment parameters
Radiation treatment planning was performed with the Varian Eclipse Treatment Planning System. In each patient, the treatment volume was delineated using post-contrast thin-slice (1-mm) gadolinium-enhanced T1-weighted and T2-weighted MRI axial sequences fused with planning computed tomography (CT) scans of 1.2 mm acquired throughout the entire cranium. The gross tumor volume (GTV) encompassed the resection cavity and any residual tumor as seen on a contrast enhancing T1 postoperative MRI. Delineation of clinical target volume (CTV), considered to contain the microscopic disease, was carried out by adding a margin of 2 cm to the GTV (standard-CTV plan). The CTV margins were reduced to 1–3 mm around natural barriers to tumor growth (the skull, ventricles, falx, etc.), as well to allow sparing of the optic nerve/chiasm, if necessary. The CTVs were expanded by 5 mm to create the planning target volumes (PTV) to compensate for variability in treatment setup and patient motion. The prescribed dose was normalized to 100% at the isocenter and 95% isodose surface covered the PTV as the minimum dose (ICRU Report 50). Treatment was given using a Tomotherapy machine. Normal tissue was contoured to include cerebral hemispheres, hippocampi, brainstem, optic nerves, and chiasm, eyes, and cerebellum. Maximum dose was 55 Gy to the eyes, optic nerve, or chiasm, and 54 Gy to the brainstem. The treatment was performed with the Raystation Planning System. The local Institutional Review Boards approved the study.
MGMT status and MIB-1/Ki67 evaluation
We assessed the MGMT gene promoter methylation status using a methylation-specific Polymerase Chain Reaction (PCR), as previously reported [23]. Briefly, genomic DNA was extracted from paraffin-embedded tumor sections and treated with sodium bisulfite using the EZ DNA Methylation-Gold kit (HISS Diagnostics, GmbH, Freiburg, Germany). Primer sequences were used to detect methylated and unmethylated MGMT promoter sequences. PCR products were separated on 2% agarose gel. A glioma cell line with a completely methylated MGMT promoter, and peripheral blood mononucleated cells, served as positive and negative control samples, respectively. A methylation percentage of 5% was used as a cut-off value: samples with methylation < 5% and > 5% were classified as unmethylated (Unmet MGMT) and methylated (MethMGMT), respectively.
Evaluation of MIB-1 Expression: Protein expression was determined by neuropathological evaluation of biopsy or resection tissue. Immunohistochemistry was performed. In brief, heat-induced epitope retrieval was performed with either citrate or ethylenediaminetetraacetic acid (EDTA) according to the manufacturer’s protocol of the respective primary antibody. Sections were incubated for 1 h with the following primary antibodies anti-Ki-67/MiB-1 (1:200; Dako M7240, Agilent Technologies, Inc., Santa Clara, CA, USA). Sections were washed and incubated with post-block solution and horseradish peroxidase (HRP) polymer reagent according to the manufacturer’s protocol of the ZytoChem-Plus HRP Polymer Kit (Zytomed Systems GmbH, Berlin, Germany). Ki-67 Labeling Index/MIB-1 demonstrates the percentage of immunoreactive tumor cells from all tumor cells.
Statistical analysis
For data collection and analysis, we used IBM® SPSS® Statistics (version 21; IBM Corp., Armonk, NY, USA). The prevalence of investigated variables as well as the calculation of means and standard deviations was obtained by descriptive statistics. Comparison between nominal variables have been made with Chi2 test. Continuous variable correlations have been investigated with Pearson’s Bivariate correlation. Threshold of statistical significance was considered p < 0.05. Overall survival (OS) and progression-free survival (PFS) in patients with recurrent or progressive tumors were estimated using the Kaplan–Meier method calculated from the time of radiation treatment to the date of death from any cause. All tests with p < 0.05 were then included in univariate analysis (log-rank test) for comparison of survival probability. Following this, all tests with p < 0.1 were included in multivariate analysis using a Cox proportional hazards model to analyze possible dependencies. Lastly, tests with p < 0.05 in multivariate analysis were considered significant. The assessment of Ki67/MIB-1-LI as survival prognosticator was performed using software X-TILE that allows to define the best cut-off point for biomarkers with minimal p-value [24]. This is an outcome-based cut-point optimization approach that illustrates the presence of substantial tumor subpopulations and shows the robustness of the relationship between a biomarker and outcome by construction of a two-dimensional projection of every possible subpopulation.
Results
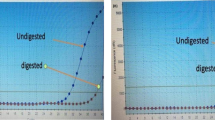
In the selected population, after a median follow-up time of 18 months [range 2–76 months], the median OS was 12 months, with 78.2% and 48.5% survival rates at 6 and 12 months, respectively. Median PFS was 7 months, with 55% and 33.6% survival rates at 6 and 12 months, respectively. Patients characteristics, age, KPS, extent of surgery, RT dose, Radiological Response, MGMT status and Ki67/MIB-1-LI and the corresponding OS data, are reported in Table 1. Regarding the MGMT promoter status, it was unmethylated in 68 cases (57.1%) and methylated in 51 (42.9%). After the survival univariate analysis (Table 1), significant factors for OS were: KPS, extent of surgical resection, RT dose, age, MGMT status, response to treatment. Moreover, we identified a most-significative cut-off value for MIB-1 of 15% of expression with a survival value (p = 0.005). The patients with a Ki67/MIB-1-LI value < 15% were 17 and had median survival 40 months, 102 patients with Ki67/MIB-1-LI value > 15% had a median survival 11 months (Fig. 1). Distribution and correlation analysis between the MIB-1 expression and other prognostic parameters showed that the MIB-1 expression level is not significantly associated with other prognostic factors: such as KPS, extent of surgery, MGMT, age and Radiological Response. On the other hand, combined MGMT status is strongly correlated to the radiological response to treatment (p = 0.000).
Multivariate analysis (Cox regression analysis) showed that KPS (HR: 3.46; 95% CI: 1.83–6.54; p = 0.000), extent of surgery (HR: 2.04; 95% CI: 1.08–3.84; p = 0.001), MGMT (HR: 2.83; 95% CI: 1.20—3.32 p = 0.023) and Ki67/MIB-1-LI status (HR: 3.85; 95% CI: 1.84—4.43; p = 0.001) were independent prognostic factors.
Interestingly, low Ki67/MIB-1-LI values were independently associated with survival, identifying long survival patients in the methylated and unmethylated patients. Indeed, the Methylated MGMT-MIB-1 < 15% group was associated with the longest OS (8 patients; median OS 41 months); Methylated MGMT-MIB-1 > 15% group (43 patients;) has a median OS 25 months (p = 0,003); in the Unmethylated MGMT-MIB-1 > 15% group (59 patients; median OS 8 months; in the Unmethylated MGMT-MIB1 < 15% group containing 9 patients median OS is not reached (Fig. 2A and B).
Discussion
Concurrent and sequential TMZ with RT, after complete surgical removal is the standard treatment for newly diagnosed GBMs. The overall expected 5-year survival rate for GBM patients is < 5% [25], and several data suggest that survival depends on a combination of intrinsic patient characteristics and genetic mutations.
In neuro-oncology, the Ki67/MIB-1-LI is widely used [21], with the expression of Ki 67/Mib-1 ≥ 10% e IDHwt strongly suggestive of GBM diagnosis. However, the prognostic role of Ki-67/MIB-1-LI remains largely debated, with large discrepancies [10,11,12,13,14,15,16,17,18,19], potentially depending on the inter- and intra-observer variability [26,27,28], and lack of standardization in the immunostaining procedure [29]. Furthermore, the prognostic role of Ki-67/MIB-1-LI has been often investigated considering other prognostic factors, rather than directly analyzing the correlation with OS [20], and only few papers approached the association considering histological heterogeneity [30, 31]. Therefore, nowadays, it is not yet a cut-off point for Ki67/MIB-1-LI capable of having a potential prognostic effect [19, 31, 32]. In the present work we demonstrate a prognostic significance for Ki67/MIB-1-LI in IDHwt GBM patients, with a cut off level 15%. The incidence of IDHwt GBMs with a Ki-67/Mib-1-LI lower than 15% are quite rare and is found only in 17 patients among 119 patients but is strongly and independently associated with a long survival with a median survival of 40 months. Thus, our evidence confirms what has already been previously described [19], with the important difference that our evidence indicates a survival of 40 months and not 18 months previously indicated [19].
Notably, when combined with MGMT status, Ki67/MIB-1-LI correlates with a higher OS of IDHwt patients, independently from MGMT promoter status. The correlation analysis didn’t clarify the modality in which low Ki67/MIB-1-LI provides a better prognosis, indeed it wasn’t correlated with any analyzed prognostic factors, nor to the radiological treatment response. In consideration of a homogeneity of treatment for the patients selected in the work, we believe that an explanatory hypothesis could be that the parameter ki/67/MIB-1 is not only a prognostic factor but also a predictive factor of response to radio-chemotherapy treatment.
Conclusions
Ki67/MIB-1-LI used with a cut-off value of 15% seems to be very interesting as a prognostic-related index in IGHwt patients, identifying those candidates to have a higher OS, independently by MGMT status. The retrospective analysis setting, the mono-centric data and, particularly, the uneven group sizes are the main limitations of the present work. Our study results present an interesting finding that warrants further investigation, perhaps in the first instance through larger retrospective studies involving multiple cancer treatment and pathology centers. More data should be collected in a prospective and multi centric setting to overcome the discrepancy of Mib-1 expression assessment due to inter-and intra-observer variability.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Wu A, Ugiliweneza B, Wang D et al (2022) Trends and outcomes of early and late palliative care consultation for adult patients with glioblastoma: a seer-medicare retrospective study. Neurooncol Pract 9:299–309. https://doi.org/10.1093/nop/npac026
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. https://doi.org/10.1056/NEJMoa043331
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 Mutations in Gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Amelot A, De Cremoux P, Quillien V et al (2015) IDH-mutation is a weak predictor of long-term survival in glioblastoma patients. PLoS ONE. https://doi.org/10.1371/journal.pone.0130596
Li LT, Jiang G, Chen Q, Zheng JN (2015) Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol Med Rep 11:1566–1572. https://doi.org/10.3892/mmr.2014.2914
Veronese SM, Maisano C, Scibilia J (1995) Comparative prognostic value of Ki-67 and MIB-1 proliferation indices in breast cancer. Anticancer Res 15:2717–2722
Thotakura M, Tirumalasetti N, Krishna R (2014) Role of Ki-67 labeling index as an adjunct to the histopathological diagnosis and grading of astrocytomas. J Cancer Res Ther 10:641. https://doi.org/10.4103/0973-1482.139154
Moskowitz SI, Jin T, Prayson RA (2006) Role of MIB1 in predicting survival in patients with glioblastomas. J Neurooncol 76:193–200. https://doi.org/10.1007/s11060-005-5262-1
Chen W-J, He D-S, Tang R-X et al (2015) Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev 16:411–420. https://doi.org/10.7314/APJCP.2015.16.2.411
Reavey-Cantwell JF, Haroun RI, Zahurak M et al (2001) The prognostic value of tumor markers in patients with glioblastoma multiforme: analysis of 32 patients and review of the literature. J Neurooncol 55:195–204. https://doi.org/10.1023/a:1013845004294
Dahlrot RH, Bangsø JA, Petersen JK et al (2021) Prognostic role of Ki-67 in glioblastomas excluding contribution from non-neoplastic cells. Sci Rep 11:17918. https://doi.org/10.1038/s41598-021-95958-9
Wong E, Nahar N, Hau E et al (2019) Cut-point for Ki-67 proliferation index as a prognostic marker for glioblastoma. Asia Pac J Clin Oncol 15:5–9. https://doi.org/10.1111/ajco.12826
Yu Z, Zhou Z, Xu M et al (2023) Prognostic factors of gliosarcoma in the real world: a retrospective cohort study. Comput Math Methods Med 2023:1–14. https://doi.org/10.1155/2023/1553408
Kumar N, Elangovan A, Madan R et al (2021) Impact of Immunohistochemical profiling of glioblastoma multiforme on clinical outcomes: real-world scenario in resource limited setting. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2021.106726
Madhugiri VS, Moiyadi AV, Shetty P et al (2021) Analysis of factors associated with long-term survival in patients with glioblastoma. World Neurosurg 149:e758–e765. https://doi.org/10.1016/j.wneu.2021.01.103
Liang J, Lv X, Lu C et al (2020) Prognostic factors of patients with gliomas – an analysis on 335 patients with glioblastoma and other forms of gliomas. BMC Cancer 20:35. https://doi.org/10.1186/s12885-019-6511-6
Bredel M, Piribauer M, Marosi C et al (2002) High expression of DNA topoisomerase IIα and Ki-67 antigen is associated with prolonged survival in glioblastoma patients. Eur J Cancer 38:1343–1347. https://doi.org/10.1016/S0959-8049(02)00065-5
Dumke R, Dumke C, Eberle F et al (2022) Monocentric evaluation of Ki-67 labeling index in combination with a modified RPA score as a prognostic factor for survival in IDH-wildtype glioblastoma patients treated with radiochemotherapy. Strahlenther Onkol 198:892–906. https://doi.org/10.1007/s00066-022-01959-6
Alkhaibary A, Alassiri AH, AlSufiani F, Alharbi MA (2019) Ki-67 labeling index in glioblastoma; does it really matter? Hematol Oncol Stem Cell Ther 12:82–88. https://doi.org/10.1016/j.hemonc.2018.11.001
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Tini P, Nardone V, Pastina P et al (2019) Is a reduction of radiation dose feasible in patients affected by glioblastoma undergoing radio-chemotherapy according to MGMT promoter methylation status without jeopardizing survival? Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2019.105445
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-Tile. Clin Cancer Res 10:7252–7259. https://doi.org/10.1158/1078-0432.CCR-04-0713
Krex D, Klink B, Hartmann C et al (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606. https://doi.org/10.1093/brain/awm204
Polley M-YC, Leung SCY, McShane LM et al (2013) An international Ki67 reproducibility study. J Nat Cancer Inst 105:1897–1906. https://doi.org/10.1093/jnci/djt306
Grzybicki DM, Liu Y, Moore SA et al (2001) Interobserver variability associated with the MIB-1 labeling index. Cancer 92:2720–2726. https://doi.org/10.1002/1097-0142(20011115)92:10%3c2720::AID-CNCR1626%3e3.0.CO;2-Z
Nielsen LAG, Bangsø JA, Lindahl KH et al (2018) Evaluation of the proliferation marker Ki-67 in gliomas: Interobserver variability and digital quantification. Diagn Pathol 13:38. https://doi.org/10.1186/s13000-018-0711-2
Aung TN, Acs B, Warrell J et al (2021) A new tool for technical standardization of the Ki67 immunohistochemical assay. Mod Pathol 34:1261–1270. https://doi.org/10.1038/s41379-021-00745-6
Henker C, Kriesen T, Schneider B et al (2019) Correlation of Ki-67 index with volumetric segmentation and its value as a prognostic marker in glioblastoma. World Neurosurg 125:e1093–e1103. https://doi.org/10.1016/j.wneu.2019.02.006
Geisenberger C, Mock A, Warta R et al (2015) Molecular profiling of long-term survivors identifies a subgroup of glioblastoma characterized by chromosome 19/20 co-gain. Acta Neuropathol 130:419–434. https://doi.org/10.1007/s00401-015-1427-y
Armocida D, Frati A, Salvati M et al (2020) Is Ki-67 index overexpression in IDH wild type glioblastoma a predictor of shorter progression free survival? A clinical and Molecular analytic investigation. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2020.106126
Funding
Open access funding provided by Università degli Studi di Siena within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
P.T and F.M wrote the main manuscript text. G.M. performed statistical analysis. C.M. performed ki-67/mib1 evaluation. All authors reviewed and correct the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Research involving human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tini, P., Yavoroska, M., Mazzei, M.A. et al. Low expression of Ki-67/MIB-1 labeling index in IDH wild type glioblastoma predicts prolonged survival independently by MGMT methylation status. J Neurooncol 163, 339–344 (2023). https://doi.org/10.1007/s11060-023-04342-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04342-2