Abstract
Purpose
Brain invasion in meningiomas is considered an indicator of more aggressive behavior and worse prognosis. But the precise definition and the prognostic role of brain invasion remains unsolved duo to lacking a standardized workflow of surgical sampling and the histopathological detection. Searching for molecular biomarker expression correlating with brain invasion, could contribute to establish a molecular pathological diagnosis without problems of subjective interobserver variation and deeply understand the mechanism of brain invasion and develop innovative therapeutic strategies.
Methods
We utilized liquid chromatography tandem mass spectrometry to quantify protein abundances between non-invasive meningiomas (n = 21) and brain-invasive meningiomas (n = 21) spanning World Health Organization grades I and III. After proteomic discrepancies were analyzed, the 14 most up-regulated or down-regulated proteins were recorded. Immunohistochemical staining for glial fibrillary acidic protein and most likely brain invasion-related proteins was performed in both groups.
Results
A total of 6498 unique proteins were identified in non-invasive and brain-invasive meningiomas. Canstatin expression in the non-invasive group was 2.1-fold that of the brain-invasive group. The immunohistochemical staining showed canstatin expressed in both groups, and the non-invasive group showed stronger staining for canstatin in the tumor mass (p = 0.0132) than the brain-invasive group, which showed moderate intensity.
Conclusion
This study demonstrated the low expression of canstatin in meningiomas with brain invasion, a finding that provide a basis for understanding the mechanism of brain invasion of meningiomas and may contribute to establish molecular pathological diagnosis and identify novel therapeutic targets for personalized care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most of meningiomas are slow-growing benign tumor and do not disrupt the surrounding brain tissue. Complete microsurgical excision is sufficient for curing the majority of the patients and contribute to good prognosis. But a subset of meningiomas have brain-invasive behavior [1] and worse prognosis [2]. Even after Simpson I resections, brain-invasive meningiomas have higher incidence of recurrence. As the clinical importance of brain invasion (BI) of meningiomas increased, it is believed being independently correlated with recurrence and became a stand-alone criterion for grade II meningiomas in the 2016 WHO classification of central nervous system (CNS) tumors [1,2,3,4]. But its prognostic impact remains controversial, based on contradictory results from various studies [5,6,7,8]. Inconstant assessment of BI from non-standardized tumor sampling and no clear-cut histopathological detection criteria may be a major key point causing the debate [2, 6, 9, 10]. In order to avoid the problem of non-standardized process in histopathological diagnosis, it is promising to establish molecular diagnosis by focusing on molecular mechanism and searching for molecular biomarker that could correlate with brain invasion.
BI in meningiomas involves molecular alterations at various cellular components and in signal transmission pathways which is related degradation of extracellular matrix/basement membrane (ECM/BM), and tumor cells migration and adhesion. By proteomic analysis, such as liquid chromatography–mass spectrometry (LC-MS/MS), these molecular alterations can be identified as biomarkers of BI of meningioma and therapeutic targets. It could contribute to establish molecular diagnosis to avoid the problematic sampling of histopathological investigation.
So, this study aimed to find difference of protein molecular expression between non-invasive and brain-invasive meningiomas using LC-MS/MS-based proteomics and try to contribute to molecular pathological diagnosis of BI and targeting therapy.
Materials and methods
We identified data from patients with histopathological diagnosis of meningioma obtained from the Department of Neurosurgery of the TangShan GongRen Hospital between 2005 and 2021. Among them, we selected 21 cases with meningiomas grade III as invasive meningioma group that are characterized by aggressive behavior presenting as loss of cerebrospinal fluid (CSF) cleft, peritumoral edema in preoperative MRI, the remarkable adhesion and invasion of surrounding brain tissues in intraoperative findings, pathologically reported meningioma cell invading into adjacent brain, while the other 21 cases with convex meningiomas grade I as non-invasive group that are characterized by well-circumscribed CSF cleft in preoperative MRI, and complete arachnoidal interface in intraoperative findings, pathologically reported no BI when sampling the brain-tumor interface of adhesion areas. All samples were stored in liquid nitrogen immediately after removal.
Immunoprecipitation
The samples used for the LC-MS/MS were all tumor tissue and did not contain any peripheral areas with a predominant proportion of normal brain tissue. Tissue mixtures were lysed in five volumes of lysis buffer (25 mM Tris-HCl, 150 mM NaCl, 3 mM MgCl2, 5% glycerol, 0.5% Nonidet P-40, 1 mM dithiothreitol, 1% protein inhibitor [PI], pH 7.4) for 2 h with rotation at 4 °C. Tissue lysates were cleared by centrifugation at 21,000 g for 30 min, followed by measurement of the supernatant using a 2-D quantitative kit. Equal amounts of protein were incubated with the ANTI-FLAG M2 affinity gel (Sigma) at 4 °C overnight, washed thrice with a wash buffer (25 mM Tris-HCl, 150 mM NaCl, 3 mM MgCl2, 0.2 mM EDTA, 0.1% Tween-20, 5% glycerol, 1% PI, pH 7.4) for 10 min, and proteins were eluted with 20 µL elution buffer containing 400 µg/mL 3×FLAG peptides (ChinaPeptides Co., Ltd.).
Immunoprecipitated proteins were separated by 12.5% SDS-PAGE gel and visualized using a Silver Staining Kit (Beyotime). The gels were de-stained and dehydrated, and the proteins were digested using sequencing-grade trypsin (Promega). The peptides were extracted from gel pieces with 0.1% formic acid (FA) and 50% acetonitrile and dried in a vacuum centrifuge (Thermo Fisher Scientific).
The peptides were dissolved in 10 µL of 0.2% FA and separated using an online Nano-LC system (Microtech Scientific) equipped with a C18 reverse-phase column. The column was eluted using a linear gradient of 5–30% acetonitrile in 0.2% FA at a rate of 500 nL/min for 100 min. Mass spectra were acquired using an LTQ-Orbitrap mass spectrometer (Thermo Fisher) equipped with a nano-ES ion source (Proxeon Biosystems). Full scan spectra (from m/z 300–1600) were acquired in an Orbitrap analyzer with a resolution of 60,000 at 400 m/z after the accumulation of 1,000,000 ions. The five most intense ions per scan were selected for collision-induced dissociation fragmentation in the linear ion trap after the accumulation of 3000 ions. We set the maximal filling times to 500 ms for the full scans and 150 ms for the LC-MS/MS scans. The dynamic exclusion list was restricted to a maximum of 500 entries, with a maximum retention period of 60 s and a relative mass window of 10 ppm.
After proteomic discrepancies were analyzed between the two groups, the 14 most up-regulated or down-regulated proteins in the non-invasive group were record. Among them, we chose most likely BI-related protein to stain immunohistochemically.
Immunohistochemical staining
Immunohistochemistry (IHC) studies were performed in formalin-fixed, paraffin-embedded tissues. Consecutive 3-µm-thick sections were cut from the recipient blocks and transferred to poly-L-lysine-coated slides for IHC analysis. A modification of heat-induced epitope retrieval, involving pre-heating of EnVision FLEX Target Retrieval low pH solution to 65 °C and incubating slide for 20 min at 97 °C, followed by natural cooling to 65 °C, was used to detect the invasion-related proteins. Endogenous peroxidase activity was blocked by incubating in methanol with 0.3% H2O2 for 20 min. The sections were blocked for 60 min with 5% normal goat serum and subsequently incubated with primary antibody against the invasion-related proteins at 4 °C overnight. The antibodies and their working dilutions were as follows: Ki-67 antibody (ab15580, 1:200; Abcam Cambridge, MA, USA), glial fibrillary acidic protein (GFAP) antibody (ab7260, 1:1000; Abcam Cambridge, MA, USA) and invasion-related proteins (identified as canstatin after LC-MS/MS) antibody (ab125208, 1:1000; Abcam Cambridge, MA, USA). After washed with Tris-buffered saline, the sections were incubated in biotinylated link (Dako) for 60 min. Next, the sections were incubated in streptavidin-HRP (Dako) for 30 min at room temperature and then expression of the invasion-related proteins was visualized by a liquid DAB + substrate chromogen system (Dako).
All the staining results were positive, and were assessed by three independent pathologists, by considering the staining color value and average positive staining area percentage (APSAP). The results are divided into three levels: 1 for weak staining, 2 for moderate staining, and 3 for strong staining.
Data analysis
All raw files were processed using the MaxQuant software (version 1.3.0.5). The generated peak list files were searched against the UniProt protein sequence database (released 2013.08 https://www.uniprot.org/). The search parameters were set as follows: enzyme selected was trypsin, with up to two missed cleavages, carbamidomethyl cysteine as a fixed modification, and methionine oxidation and protein N-terminal acetylation as variable modifications. The MS tolerance was 6 ppm, while the MS/MS tolerance was 0.5 Da. The required false discovery rate was set to 1% at the peptide and protein levels, and the minimum required peptide length was seven amino acids. At least one unique or razor peptide per protein group was required for protein identification.
For IHC statistical analysis: A chi-square test (SSPS, version 11.0; SPSS, Inc., Chicago, IL, USA) was used to determine the significance of the association between the two groups. Differences were considered statistically significant at P < 0.05.
Results
A total of 6498 unique proteins were identified (Supplement 1). Proteomic differences were observed between the two groups (Fig. 1). The 14 most up-regulated proteins in the non-invasive group were P10915, P03973, Q01469, O95050, P15259, Q4V9L6, P04733, Q30134, P02745, P08572, Q8IZR5, P08473, P28906 and E7EX88(Table 1). The 14 most down-regulated proteins were P17600, P14136, Q9H0Q3, Q9UQM7-2, P69905, Q16352, 95741-2, P62760, Q05315, Q13268-2, P07197, P13746-2, P02686 and Q92686 (Table 2). Among these proteins, we found that canstatin (P08572) probably related to BI and then performed immunohistochemistry study on it.
The immunohistochemical staining results of these portions showed canstatin expression in the both groups. All the meningiomas showed positive expression of canstatin, and in non-invasive group (Avarage ± STDEV; 2.35 ± 0.74) it showed strong staining for canstatin (p = 0.0132) (Figs. 2 and 3) compared to brain-invasive group (Avarage ± STDEV; 1.75 ± 0.72), which showed moderate intensity in the tumor mass.
The average of Ki-67 expression was 27 ± 6.13% in the brain-invasive group, and 2.32 ± 0.56% in the none-invasive group of meningiomas.
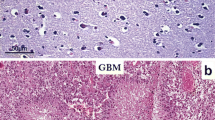
Immunohistochemical study for immune intensity of brain-tumor interface and representative staining for non-invasive and brain-invasive meningiomas. Immunohistochemical results are analyzed by determining the staining color value and average positive staining area percentage. All staining results are positive. Results are divided into three levels: 1 for weak staining (A), 2 for moderate staining (B), and 3 for strong staining (C). D, E, and F Non-invasive group (HE; canstatin, and GFAP), which shows strong staining for canstatin in the tumor mass (E). G, H, and I Brain-invasive group (HE; canstatin, and GFAP), which shows moderate intensity in tumor mass (H)
Disccusion
This study found the low expression of canstatin in meningiomas with BI. It provided a basis for understanding the molecular mechanism of BI of meningiomas and may contribute to establish molecular pathological diagnosis and identify novel therapeutic targets.
Standard for BI in meningiomas
Systematic and accurate detection standard for BI in meningiomas contains pre-, intra-, and post-operative methods. That is imaging, intraoperative and histopathological assessment [2, 11]. BI by meningioma is defined as tongue-like protrusions of tumor cells into underlying GFAP-positive cortical parenchyma, without intervening leptomeningeal layer at the tumor-CNS interface [12]. Although at present, histopathological examination is the only standard for diagnosing BI, there is no standardized way of surgical sampling to ensure the accuracy of the detection of BI in neuropathological analysis and no clear-cut criteria exist for the histopathological detection of BI. Many studies have investigated the correlation between preoperative radiological features and BI [13,14,15,16], such as peritumoral oedema, heterogeneous contrast enhancement, and irregular tumor shape, disruption of arachnoid at the brain tumor interface, enlarged pial feeding arteries, which have been identified as predictors of BI [13,14,15,16]. In particular, edema volume was significantly and statistically related to brain-invasive meningioma [15]. However, duo to lack of definite criteria of histopathological detection of BI as a reference, there is no established radiographic criteria that clearly depicts BI. The additional intraoperative assessment regarding BI by neurosurgeon might be of clinical value for a more precise assessment of this tumor characteristic, especially in cases of incomplete sampling. But, even though under high magnification the breaching of the intervening leptomeningeal surface by meningioma tissue is detectable, it is impossible to see whether meningioma cells protrude into brain tissue. On the other hand, the intraoperative assessment is clearly prone to interobserver variance depending on the surgeons’ experience. In this study, we adopt a stricter criterion including preoperative imaging, intraoperative and histopathological assessment in order to avoid minimal histopathological criteria for calling BI in questionable samples, optimize interobserver reproducibility and ensure every invasive sample should be irrefutable before assigning.
The role of BI in prognosis and grading
BI in meningioma is not only associated with surgical decision-making but is also independently associated with recurrence and poor prognosis [2]. But the precise definition and the prognostic role of BI remains highly controversial [5, 6, 8]. But even so, BI in meningioma was clearly defined as an additional criterion for atypia in the revised fourth edition of the 2016 WHO classification of CNS tumors [17]. The 2021 WHO criteria kept BI as a standalone diagnostic feature for grade 2 meningiomas [18]. Because the majority of brain-invasive meningiomas also show other high-grade (atypical) features (such as elevated mitotic activity) and BI in previous grade II and III tumors is no longer disputed, only a subset of BI otherwise benign meningiomas, that is meningiomas classified as grade 2 solely on the presence of BI, remains controversial. Since many changes occurred in the WHO criteria in this decades, it must confound interpretation of meningioma grading in this study (2005–2021). That is earlier grade I meningiomas likely contained many grade II samples in this study. In order to eliminate this confusion of grading, we only collect invasive cases in WHO grade III meningiomas met invasive criterion and collect non-invasive cases in non-invasive grade I meningiomas to exclude WHO grade II meningiomas which may include a subset of BI otherwise benign meningiomas with controversial.
However, inconstant assessment of BI may be a major key point causing previous debates about the prognosis value of BI [2, 6]. Intraoperative tumor sampling is non-standardized and especially areas of interest may not always be amenable to appropriate sampling [11].
There are several factors that can potentially influence the intraoperative sampling. In cases of skull base meningiomas, the trend of performing smaller and more focused surgical approaches will very likely contradict optimal intraoperative conditions for sampling. When meningiomas is adjacent to highly eloquent areas, especially if adhesions or possibly infiltrative growth is present, it is most important for neurosurgeons to leave the arachnoid membrane intact to avoid damaging cortex and do not expand sampling to include bordering cortical tissue [19]. If no brain tissue is detectable, evaluation of BI is not possible. In neurosurgical practice, meningiomas are usually resected piece-meal by suction and Cavitron Ultrasonic Surgical Aspirator (CUSA). The use of CUSAs, with subsequent tissue loss may further lead to the difficulty in selective sampling of the interface in meningioma tissue and no dedicated specimen from the tumor surface for neuropathological analysis was obtained [20]. It is documented that the more specimens available, the more BI observed [21].
On the other hand, the histopathological characteristics used to determine BI are not clearly defined [2]and possibly vary between departments and neurooncological centers. Further, variations in interobserver interpretation and different staining protocols make it difficult to establish clear cut off values.
Molecular mechanisms of brain-invasive and LC-MS/MS-based proteomic
Since BI and grading of meningiomas is based on subjective assessment of histopathological findings, this system is suboptimal with problematic interobserver variation. Advances in molecular characterization of meningiomas have revealed several genetic aberrations and driver mutations. Molecular classification of meningiomas based on copy number variation, point mutations, methylation, and transcriptomic and proteomic data stands out as a future diagnostic work-up of meningiomas [22]. Thus, WHO CNS 5th endorses molecular biomarkers to refine classification and malignancy grading. Some studies have focused on molecular mechanism and searching for molecular biomarker expression that could correlate with BI, in order to avoid subjective assessment of histopathological findings with problems of interobserver variation.
It has been documented that BI in meningioma is correlated to molecular alterations at various cellular components and in signal transmission pathways [23]. Such alterations result in three-step process, initially degradation of ECM/BM, and tumor cells migration, finally promoting adhesion of meningioma cells to resident cells with the help of growth factors and blood-vessel formation [24], leading to the tendency of the tumor to infiltrate and local BI.
Since BI related molecular alterations depend on protein dynamics, demonstrating changes from the proteomic perspective enables us to understand mechanism of the BI. From body fluids analysis, proteomics could identify possible biomarkers of BI, such as those proteins secreted by pathological cells or affected by BI processes. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the standard laboratory technique for the analysis of biological fluids. It has reached maturity so that most small-molecule concentrations in the lower picomolar range can be successfully assessed. In this study, LC-MS/MS-based proteomics showed that canstatin was down-regulated in the brain-invasive group, which indicated that canstatin may contribute to the inhibition of BI.
The role of canstatin in inhibition of BI
In fact, canstatin is closely related to ECM/BM-one of the components of BI. Basement membrane is mainly composed of type IV collagen which has been recently identified being involved in the regulation of tumor angiogenesis [25]. Type IV collagen has six different α-chains, α1–α6. The triple helix of type IV collagen consisted of two α1-chains and one α2-chain is ubiquitously expressed in the basement membrane of whole body. C-terminal domain of type IV collagen called non-collagenous 1 (NC1) domain plays an important role in the assembly of α-chains [26]. During tumor progression and metastasis, the type IV collagen is degraded by ECM-degrading enzymes, such as matrix metalloproteinase (MMP)-2 and MMP-9 [25].
Canstatin is a 24 kDa non-collagenous C-terminal fragment cleaved from type IV collagen α2-chain. It was firstly identified as a recombinant protein with potent anti-angiogenic and anti-tumor activities [27]. Canstatin inhibits angiogenesis through the inhibition of proliferation, migration and tube formation in vascular endothelial cells [27], through the regulation of the Akt and FAK pathways [28]. Canstatin also induces apoptosis in vascular endothelial cells [27], by inhibiting the FAK/Akt pathway in vascular endothelial cells [29], or by activating of the Fas-dependent apoptotic pathway [28].
Currently, endogenous angiogenesis inhibitors, which are mainly proteins or fragments formed in vivo, are widely used due to their non-toxicity, lower drug resistance, high tolerance [30], particularly endogenous angiogenesis basement membrane collagen-derived inhibitors [31]. Since canstatin originates from endogenous type IV collagen α2-chain expressed in the whole body, it may have fewer side effects than the approved chemotherapeutic agents. It suggests that canstatin is not only an attractive molecular biomarker but also probably be a novel therapeutic target for BI of meningiomas.
Conclusion
Our results demonstrated the low expression of canstatin in brain-invasive meningiomas, a finding that may contribute to the development of new molecular diagnosis and therapeutic tools for the BI of meningiomas.
References
Nowosielski M, Galldiks N, Iglseder S, Kickingereder P, von Deimling A, Bendszus M, Wick W, Sahm F (2017) Diagnostic challenges in meningioma. Neuro Oncol 19:1588–1598. https://doi.org/10.1093/neuonc/nox101
Brokinkel B, Hess K, Mawrin C (2017) Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: a systematic review. Neuro Oncol 19:1298–1307. https://doi.org/10.1093/neuonc/nox071
Sun SQ, Kim AH, Cai C, Murphy RK, DeWees T, Sylvester P, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Leonard JR, Evans J, Simpson JR, Robinson CG, Perrin RJ, Huang J, Chicoine MR (2014) Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery 75:347–354. https://doi.org/10.1227/NEU.0000000000000461. (discussion 354 – 345; quiz 355)
Sun SQ, Cai C, Murphy RK, DeWees T, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Leonard JR, Evans J, Simpson JR, Robinson CG, Perrin RJ, Huang J, Chicoine MR, Kim AH (2014) Management of atypical cranial meningiomas, part 2: predictors of progression and the role of adjuvant radiation after subtotal resection. Neurosurgery 75:356–363. https://doi.org/10.1227/NEU.0000000000000462. (discussion 363)
Banan R, Abbetmeier-Basse M, Hong B, Dumitru CA, Sahm F, Nakamura M, Krauss JK, Hartmann C (2021) The prognostic significance of clinicopathological features in meningiomas: microscopic brain invasion can predict patient outcome in otherwise benign meningiomas. Neuropathol Appl Neurobiol 47:724–735. https://doi.org/10.1111/nan.12700
Baumgarten P, Gessler F, Schittenhelm J, Skardelly M, Tews DS, Senft C, Dunst M, Imoehl L, Plate KH, Wagner M, Steinbach JP, Seifert V, Mittelbronn M, Harter PN (2016) Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol 132:479–481. https://doi.org/10.1007/s00401-016-1598-1
Behling F, Fodi C, Gepfner-Tuma I, Machetanz K, Renovanz M, Skardelly M, Bornemann A, Honegger J, Tabatabai G, Tatagiba M, Schittenhelm J (2020) CNS invasion in meningioma-how the intraoperative assessment can improve the prognostic evaluation of tumor recurrence. Cancers. https://doi.org/10.3390/cancers12123620
Biczok A, Jungk C, Egensperger R, von Deimling A, Suchorska B, Tonn JC, Herold-Mende C, Schichor C (2019) Microscopic brain invasion in meningiomas previously classified as WHO grade I is not associated with patient outcome. J Neurooncol 145:469–477. https://doi.org/10.1007/s11060-019-03312-x
Backer-Grondahl T, Moen BH, Arnli MB, Torseth K, Torp SH (2014) Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol 7:7206–7219
Spille DC, Hess K, Sauerland C, Sanai N, Stummer W, Paulus W, Brokinkel B (2016) Brain Invasion in meningiomas: incidence and correlations with clinical variables and prognosis. World Neurosurg 93:346–354. https://doi.org/10.1016/j.wneu.2016.06.055
Behling F, Hempel JM, Schittenhelm J (2021) Brain invasion in meningioma—a prognostic potential worth exploring. Cancers. https://doi.org/10.3390/cancers13133259
Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM (1997) Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 21:1455–1465. https://doi.org/10.1097/00000478-199712000-00008
Friconnet G, Espindola Ala VH, Janot K, Brinjikji W, Bogey C, Lemnos L, Salle H, Saleme S, Mounayer C, Rouchaud A (2019) MRI predictive score of pial vascularization of supratentorial intracranial meningioma. Eur Radiol 29:3516–3522. https://doi.org/10.1007/s00330-019-06197-6
Huang RY, Bi WL, Griffith B, Kaufmann TJ, la Fougere C, Schmidt NO, Tonn JC, Vogelbaum MA, Wen PY, Aldape K, Nassiri F, Zadeh G, Dunn IF, International Consortium on M (2019) Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol 21:i44–i61. https://doi.org/10.1093/neuonc/noy143
Ong T, Bharatha A, Alsufayan R, Das S, Lin AW (2021) MRI predictors for brain invasion in meningiomas. Neuroradiol J 34:3–7. https://doi.org/10.1177/1971400920953417
Joo L, Park JE, Park SY, Nam SJ, Kim YH, Kim JH, Kim HS (2021) Extensive peritumoral edema and brain-to-tumor interface MRI features enable prediction of brain invasion in meningioma: development and validation. Neuro Oncol 23:324–333. https://doi.org/10.1093/neuonc/noaa190
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Raffa G, Picht T, Scibilia A, Rosler J, Rein J, Conti A, Ricciardo G, Cardali SM, Vajkoczy P, Germano A (2019) Surgical treatment of meningiomas located in the rolandic area: the role of navigated transcranial magnetic stimulation for preoperative planning, surgical strategy, and prediction of arachnoidal cleavage and motor outcome. J Neurosurg. https://doi.org/10.3171/2019.3.JNS183411
Brokinkel B, Stummer W (2016) Brain Invasion in meningiomas: the rising importance of a uniform neuropathologic assessment after the release of the 2016 world health organization classification of central nervous system tumors. World Neurosurg 95:614–615. https://doi.org/10.1016/j.wneu.2016.08.047
Pizem J, Velnar T, Prestor B, Mlakar J, Popovic M (2014) Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol 33:354–363. https://doi.org/10.5414/NP300750
Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, Macklin AM, Khan S, Singh O, Karimi S, Corona RI, Liu LY, Chen CY, Chakravarthy A, Wei Q, Mehani B, Suppiah S, Gao A, Workewych AM, Tabatabai G, Boutros PC, Bader GD, de Carvalho DD, Kislinger T, Aldape K, Zadeh G (2021) A clinically applicable integrative molecular classification of meningiomas. Nature 597:119–125. https://doi.org/10.1038/s41586-021-03850-3
Brunasso L, Bonosi L, Costanzo R, Buscemi F, Giammalva GR, Ferini G, Valenti V, Viola A, Umana GE, Gerardi RM, Sturiale CL, Albanese A, Iacopino DG, Maugeri R (2022) Updated systematic review on the role of brain invasion in intracranial meningiomas: what, when. Why? Cancers. https://doi.org/10.3390/cancers14174163
Qin C, Huang M, Pan Y, Li Y, Long W, Liu Q (2021) Brain-invasive meningiomas: molecular mechanisms and potential therapeutic options. Brain Tumor Pathol 38:156–172. https://doi.org/10.1007/s10014-021-00399-x
Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3:422–433. https://doi.org/10.1038/nrc1094
Kuhn K (1995) Basement membrane (type IV) collagen. Matrix Biol 14:439–445. https://doi.org/10.1016/0945-053x(95)90001-2
Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R (2000) Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem 275:1209–1215. https://doi.org/10.1074/jbc.275.2.1209
Panka DJ, Mier JW (2003) Canstatin inhibits akt activation and induces Fas-dependent apoptosis in endothelial cells. J Biol Chem 278:37632–37636. https://doi.org/10.1074/jbc.M307339200
Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, Griscelli F, Opolon P, Perricaudet M (2005) Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res 65:4353–4361. https://doi.org/10.1158/0008-5472.CAN-04-3536
Limaverde-Sousa G, Sternberg C, Ferreira CG (2014) Antiangiogenesis beyond VEGF inhibition: a journey from antiangiogenic single-target to broad-spectrum agents. Cancer Treat Rev 40:548–557. https://doi.org/10.1016/j.ctrv.2013.11.009
Monboisse JC, Oudart JB, Ramont L, Brassart-Pasco S, Maquart FX (2014) Matrikines from basement membrane collagens: a new anti-cancer strategy. Biochim Biophys Acta 1840:2589–2598. https://doi.org/10.1016/j.bbagen.2013.12.029
Funding
This work was supported by Hebei Provincial Department of Science and Technology (Grant No.16397747D) and the Medicine and Health Science and Technology Plan Projects in Zhejiang Province (2022KY201).
Author information
Authors and Affiliations
Contributions
JP and XX designed the research study. PL, MW and LX performed the research. LX and XX wrote the main manuscript text. YHG, BGT, DYW, YZ and ZYZ provided help and advice on this research. JP, PL, LYL,SSH and MW analyzed the data. All authors contributed to editorial changes in the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval and consent to participate
Our study protocol was approved by the ethics committee of TangShan GongRen Hospital (Ethical Number: GRYY-LL-2016-76).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pei, J., Li, P., Gao, Y.H. et al. Type IV collagen-derived angiogenesis inhibitor: canstatin low expressing in brain-invasive meningiomas using liquid chromatography–mass spectrometry (LC-MS/MS). J Neurooncol 161, 415–423 (2023). https://doi.org/10.1007/s11060-023-04256-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04256-z