Abstract
Purpose
Glioblastoma (GBM) is the most common and deadliest brain tumor with unrelenting and rapid disease progression. The standard of care for GBM is surgical excision followed by radiation with concurrent and adjuvant temozolomide-centered chemotherapy (TMZ). Treatment failure and resistance is the rule and despite advances in imaging technology, early detection of treatment failure or impending resistance remains a challenge. There is a dire, unmet, need in clinical practice for minimally-invasive diagnostic tools to enable timely understanding of disease progression and treatment response. Here, we aim to address this clinical need by leveraging a unique characteristic of GBM: the overexpression of the α2 variant of the IL-13 receptor in over 75% of GBM tumors.
Methods
In this study we examined patients with primary GBM from Penn State and Cleveland Clinic compared to healthy controls.
Results
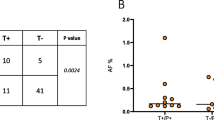
IL13Rα2 was detectable in plasma of GBM patients using ELISA but detection could be optimized by PEG precipitation to enrich for extracellular vesicles (EVs). Patients with GBM had elevated levels of plasma IL13Rα2, which correlated to levels of this receptor in the tumor tissue. Elevated plasma levels of IL13Rα2 predicted longer overall survival (OS) (19.8 vs. 13.2 months). Similarly, detection of IL13Rα2 + cells in tumor tissue also predicted longer OS (22.1 vs. 12.2 months).
Conclusion
These findings strongly suggest that expression of the IL13Rα2 receptor confer survival advantage in GBM patients, which can be determined through a minimally-invasive liquid biopsy. Detection of plasma IL13Rα2 can also be used to select GBM patients for targeted tumor therapy.




Similar content being viewed by others
References
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology 17(Suppl 4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G (2016) Immune evasion strategies of glioblastoma. Front Surg 3:11. https://doi.org/10.3389/fsurg.2016.00011
Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI (2018) Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging 2018:6828396. https://doi.org/10.1155/2018/6828396
Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, El-Rifai W, Bedognetti D, Batra SK, Haris M, Bhat AA, Macha MA (2022) Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer 21(1):79. doi:https://doi.org/10.1186/s12943-022-01543-7
Mrowczynski OD, Madhankumar AB, Sundstrom JM, Zhao Y, Kawasawa YI, Slagle-Webb B, Mau C, Payne RA, Rizk EB, Zacharia BE, Connor JR (2018) Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 9(90):36083–36101. doi:https://doi.org/10.18632/oncotarget.26300
Morad G, Carman CV, Hagedorn EJ, Perlin JR, Zon LI, Mustafaoglu N, Park TE, Ingber DE, Daisy CC, Moses MA (2019) Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano 13(12):13853–13865. https://doi.org/10.1021/acsnano.9b04397
Ebrahimkhani S, Vafaee F, Hallal S, Wei H, Lee MYT, Young PE, Satgunaseelan L, Beadnall H, Barnett MH, Shivalingam B, Suter CM, Buckland ME, Kaufman KL (2018) Deep sequencing of circulating exosomal microRNA allows non-invasive glioblastoma diagnosis. NPJ Precis Oncol 2:28. doi:https://doi.org/10.1038/s41698-018-0071-0
Batool SM, Muralidharan K, Hsia T, Falotico S, Gamblin AS, Rosenfeld YB, Khanna SK, Balaj L, Carter BS (2022) Highly sensitive EGFRvIII detection in circulating extracellular vesicle RNA of glioma patients. Clin Cancer Res 28(18):4070–4082. https://doi.org/10.1158/1078-0432.CCR-22-0444
Hori T, Sasayama T, Tanaka K, Koma YI, Nishihara M, Tanaka H, Nakamizo S, Nagashima H, Maeyama M, Fujita Y, Yokozaki H, Hirose T, Kohmura E (2019) Tumor-associated macrophage related interleukin-6 in cerebrospinal fluid as a prognostic marker for glioblastoma. J Clin Neurosci 68:281–289. doi:https://doi.org/10.1016/j.jocn.2019.07.020
Peles E, Lidar Z, Simon AJ, Grossman R, Nass D, Ram Z (2004) Angiogenic factors in the cerebrospinal fluid of patients with astrocytic brain tumors. Neurosurgery 55(3):562–567. https://doi.org/10.1227/01.neu.0000134383.27713.9a
Reategui E, van der Vos KE, Lai CP, Zeinali M, Atai NA, Aldikacti B, Floyd FP Jr, Thapar AHK, Hochberg V, Sequist FH, Nahed LV, B BV, Toner SC, Balaj M, D L, Breakefield TT, Stott XO SL (2018) Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat Commun 9(1):175. doi:https://doi.org/10.1038/s41467-017-02261-1
Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W (2002) IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia 4(5):388–399. doi:https://doi.org/10.1038/sj.neo.7900234
Thaci B, Brown CE, Binello E, Werbaneth K, Sampath P, Sengupta S (2014) Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro Oncol 16(10):1304–1312. doi:https://doi.org/10.1093/neuonc/nou045
Mintz A, Gibo DM, Madhankumar AB, Cladel NM, Christensen ND, Debinski W (2008) Protein- and DNA-based active immunotherapy targeting interleukin-13 receptor alpha2. Cancer Biother Radiopharm 23(5):581–589. doi:https://doi.org/10.1089/cbr.2008.0462
Nguyen V, Conyers JM, Zhu D, Gibo DM, Dorsey JF, Debinski W, Mintz A (2011) IL-13Ralpha2-targeted therapy escapees: biologic and therapeutic implications. Transl Oncol 4(6):390–400. https://doi.org/10.1593/tlo.11175
Zeng J, Zhang J, Yang YZ, Wang F, Jiang H, Chen HD, Wu HY, Sai K, Hu WM (2020) IL13RA2 is overexpressed in malignant gliomas and related to clinical outcome of patients. Am J Transl Res 12(8):4702–4714
Han J, Puri RK (2018) Analysis of the cancer genome atlas (TCGA) database identifies an inverse relationship between interleukin-13 receptor alpha1 and alpha2 gene expression and poor prognosis and drug resistance in subjects with glioblastoma multiforme. J Neurooncol 136(3):463–474. doi:https://doi.org/10.1007/s11060-017-2680-9
Grambsch TMTaPM (2000) Modeling survival data: extending the cox model. Springer, Berlin
Therneau TM (2020) A package for survival analysis in R
Project TJ (2022) Jamovi. https://www.jamovi.org
RCT (2021) R: A Language and environment for statistical computing (Version 4.1) [Computer Software]
Madhankumar AB, Mintz A, Debinski W (2004) Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia 6(1):15–22. doi:https://doi.org/10.1016/s1476-5586(04)80049-6
Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR (2006) Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther 5(12):3162–3169. doi:https://doi.org/10.1158/1535-7163.MCT-06-0480
Madhankumar AB, Slagle-Webb B, Wang X, Yang QX, Antonetti DA, Miller PA, Sheehan JM, Connor JR (2009) Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther 8(3):648–654. doi:https://doi.org/10.1158/1535-7163.MCT-08-0853
Liu X, Madhankumar AB, Miller PA, Duck KA, Hafenstein S, Rizk E, Slagle-Webb B, Sheehan JM, Connor JR, Yang QX (2016) MRI contrast agent for targeting glioma: interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro Oncol 18(5):691–699. doi:https://doi.org/10.1093/neuonc/nov263
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375(26):2561–2569. https://doi.org/10.1056/NEJMoa1610497
Saka M, Amano T, Kajiwara K, Yoshikawa K, Ideguchi M, Nomura S, Fujisawa H, Kato S, Fujii M, Ueno K, Hinoda Y, Suzuki M (2010) Vaccine therapy with dendritic cells transfected with Il13ra2 mRNA for glioma in mice. J Neurosurg 113(2):270–279. doi:https://doi.org/10.3171/2009.9.JNS09708
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, Organisation European, for R, Treatment of Cancer Brain Radiation Oncology T, (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Zhao Z, Wijerathne H, Godwin AK, Soper SA (2021) Isolation and analysis methods of extracellular vesicles (EVs). Extracell Vesicles Circ Nucl Acids 2:80–103. doi:https://doi.org/10.20517/evcna.2021.07
Gamez-Valero A, Monguio-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borras FE (2016) Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci Rep 6:33641. https://doi.org/10.1038/srep33641
Chen W, Sivaprasad U, Tabata Y, Gibson AM, Stier MT, Finkelman FD, Hershey GK (2009) IL-13R alpha 2 membrane and soluble isoforms differ in humans and mice. J Immunol 183(12):7870–7876. doi:https://doi.org/10.4049/jimmunol.0901028
Newman JP, Wang GY, Arima K, Guan SP, Waters MR, Cavenee WK, Pan E, Aliwarga E, Chong ST, Kok CYL, Endaya BB, Habib AA, Horibe T, Ng WH, Ho IAW, Hui KM, Kordula T, Lam PYP (2017) Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nat Commun 8(1):1913. doi:https://doi.org/10.1038/s41467-017-01392-9
Kefayat A, Amouheidari A, Ghahremani F, Alirezaei Z (2021) Diagnostic and prognostic value of stem cell factor plasma level in glioblastoma multiforme patients. Cancer Med 10(15):5154–5162. doi:https://doi.org/10.1002/cam4.4073
Kumar DM, Thota B, Shinde SV, Prasanna KV, Hegde AS, Arivazhagan A, Chandramouli BA, Santosh V, Somasundaram K (2010) Proteomic identification of haptoglobin alpha2 as a glioblastoma serum biomarker: implications in cancer cell migration and tumor growth. J Proteome Res 9(11):5557–5567. doi:https://doi.org/10.1021/pr1001737
Iwamoto FM, Hottinger AF, Karimi S, Riedel E, Dantis J, Jahdi M, Panageas KS, Lassman AB, Abrey LE, Fleisher M, DeAngelis LM, Holland EC, Hormigo A (2011) Serum YKL-40 is a marker of prognosis and disease status in high-grade gliomas. Neuro Oncol 13(11):1244–1251. doi:https://doi.org/10.1093/neuonc/nor117
Schuhmann MU, Zucht HD, Nassimi R, Heine G, Schneekloth CG, Stuerenburg HJ, Selle H (2010) Peptide screening of cerebrospinal fluid in patients with glioblastoma multiforme. Eur J Surg Oncol 36(2):201–207. doi:https://doi.org/10.1016/j.ejso.2009.07.010
Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y (2003) Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res 63(20):6962–6970
Buccitelli C, Selbach M (2020) mRNAs, proteins and the emerging principles of gene expression control. Nat Rev Genet 21(10):630–644. doi:https://doi.org/10.1038/s41576-020-0258-4
Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A (2006) IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 12(1):99–106. doi:https://doi.org/10.1038/nm1332
Saikali S, Avril T, Collet B, Hamlat A, Bansard JY, Drenou B, Guegan Y, Quillien V (2007) Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol 81(2):139–148. doi:https://doi.org/10.1007/s11060-006-9220-3
Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D’Apuzzo M, Barish ME, Forman SJ, Jensen MC (2012) Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res 18(8):2199–2209. doi:https://doi.org/10.1158/1078-0432.CCR-11-1669
Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA, Strober W (2008) Restoration of tumor immunosurveillance via targeting of interleukin-13 receptor-alpha 2. Cancer Res 68(9):3467–3475. doi:https://doi.org/10.1158/0008-5472.CAN-07-5301
Han J, Yang L, Puri RK (2005) Analysis of target genes induced by IL-13 cytotoxin in human glioblastoma cells. J Neurooncol 72(1):35–46. doi:https://doi.org/10.1007/s11060-004-3119-7
Pointer KB, Clark PA, Schroeder AB, Salamat MS, Eliceiri KW, Kuo JS (2017) Association of collagen architecture with glioblastoma patient survival. J Neurosurg 126(6):1812–1821. doi:https://doi.org/10.3171/2016.6.JNS152797
Aggarwal A, Herz N, Campbell P, Arkush L, Short S, Rees J (2015) Diagnostic delay and survival in high-grade gliomas - evidence of the ‘waiting time paradox’? Br J Neurosurg 29(4):520–523. doi:https://doi.org/10.3109/02688697.2015.1012050
Zhang M, Xu F, Ni W, Qi W, Cao W, Xu C, Chen J, Gao Y (2020) Survival impact of delaying postoperative chemoradiotherapy in newly-diagnosed glioblastoma patients. Transl Cancer Res 9(9):5450–5458. doi:https://doi.org/10.21037/tcr-20-1718
Sun MZ, Oh T, Ivan ME, Clark AJ, Safaee M, Sayegh ET, Kaur G, Parsa AT, Bloch O (2015) Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg 122(5):1144–1150. doi:https://doi.org/10.3171/2014.9.JNS14193
Knudson KM, Hwang S, McCann MS, Joshi BH, Husain SR, Puri RK (2022) Recent advances in IL-13Ralpha2-directed cancer immunotherapy. Front Immunol 13:878365. https://doi.org/10.3389/fimmu.2022.878365
Acknowledgements
The authors acknowledge the Penn State Neuroscience Institute Biorepository and the Cleveland Clinic for providing tissue samples and clinical data crucial for this study. This work was partially supported by the Tara Leah Witmer Fund. The results shown here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by VK, ET, and DN. Preparation and analysis of clinical data was performed by TC, AD, TB, KW, and NS. GS, BP, GB, OM, and BZ contributed to the study conception, design, and implementation. JL, JBS, and JC provided guidance and oversight of the project. The first draft of the manuscript was written by VK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have not disclosed any competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khristov, V., Nesterova, D., Trifoi, M. et al. Plasma IL13Rα2 as a novel liquid biopsy biomarker for glioblastoma. J Neurooncol 160, 743–752 (2022). https://doi.org/10.1007/s11060-022-04196-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04196-0